Progressive cognitive impairments are a hallmark of Alzheimer’s Disease (AD), but the timing of their onset and progression varies by patient. Even among people with the typical amnestic form of AD, the speed with which the disease progresses can vary significantly: some patients experience rapid cognitive decline, while others deteriorate over a decade or more.
The unpredictability of AD’s pace creates uncertainty and stress for both patients and their caregivers. It is also a risk to clinical trials, since the success of a clinical trial depends on being able to measure a treatment effect over a set period of time (usually six months for Phase II). Too many slow progressors in a trial cohort can cause a study to fail, even if the drug is effective.
The Role of Tau in AD Progression
A group of researchers from Massachusetts General Hospital explored whether molecular variations in tau species — specifically those that influence its propagation — explain AD progression. According to their research paper, “Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s Disease,” published in the June 2020 issue of Nature Medicine, tau propagation partially explains how and why the pace of AD’s development in patients varies so significantly.
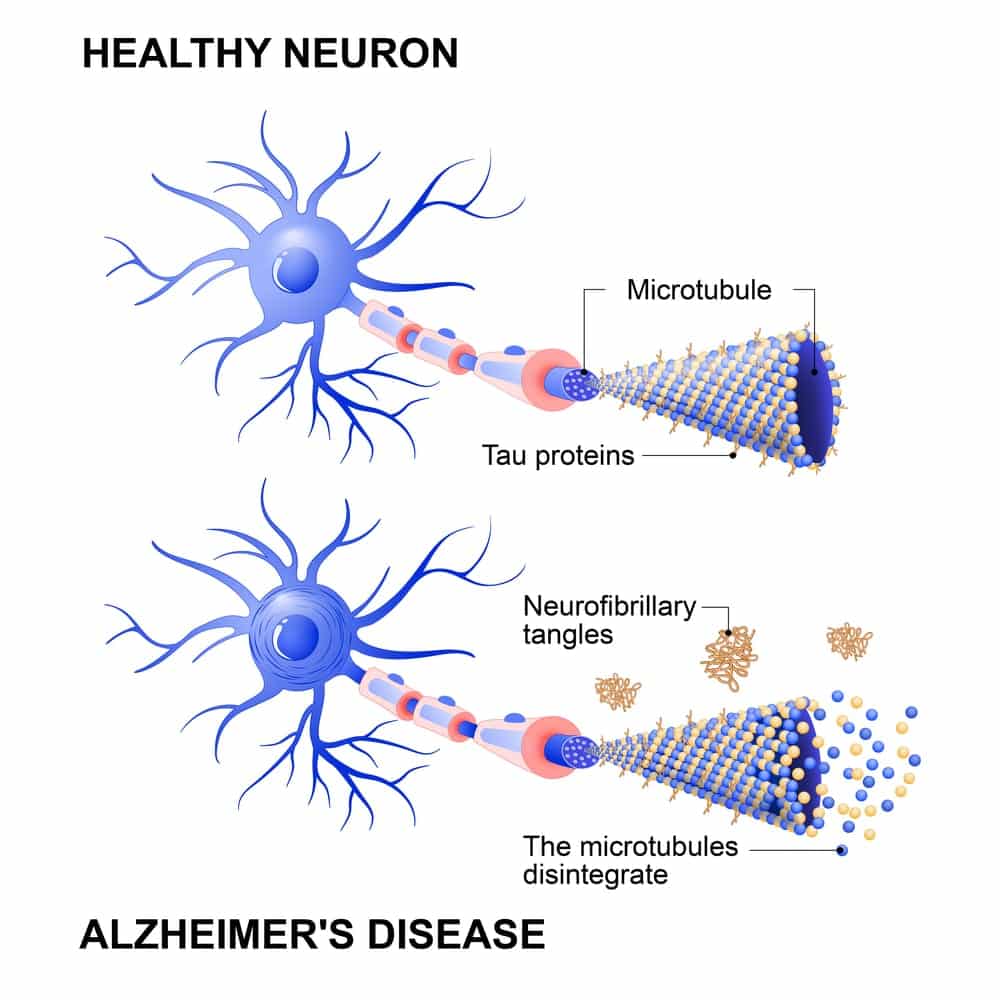
Tau is a small protein that can build up in the brain, damaging cells essential for learning and memory — which means it plays a major role in a host of brain diseases. Tau buildup is caused by increased activity of enzymes that act on tau, called tau kinases. This increased enzymatic activity causes the tau proteins to misfold, forming neurofibrillary tangles (NFTs), which then pervade the brain in a standard sequence. According to the researchers’ findings, the propagation of these tau tangles is closely tied to AD’s clinical progression.
Research Findings
Looking at postmortem brain samples from thirty-two people who died with advanced AD, the researchers found significant variability in tau’s ability to seed aggregation. At the time of death, each brain examined had extensive NFTs, evaluated as Braak stage V/VI (mid-pathologic/advanced stage). Braak staging focuses on the location of NFT:
- Stages I and II indicate that NFTs are limited to the transentorhinal region of the brain.
- Stages III and IV occur when NFTs are in the limbic regions.
- In Stages V and VI, NFTs are found extensively in the neocortical regions of the brain.
Although all postmortem brains examined were Braak stages V/VI, the patients’ clinical trajectories had been variable. Age at onset was 45–81, and time between onset and death was between five and 19 years. The patients’ rate of cognitive decline as gauged by serial tests on the Clinical Dementia Rating Sum of Boxes (CDR-SOB) varied widely.
The team measured the capacity for tau to seed aggregation in biosensor cell lines. When equipped with a tau fragment tagged with fluorescent donor and acceptor molecules, these cells light up when potent seeds spark aggregation. Despite the fact, that the researchers normalized the amount of tau used from each brain, they found a wide variation in seeding activity — ranging by an order of magnitude across samples. Three samples with the highest seeding activity came from people who carried two copies of ApoE4, which suggests that this risk factor influences tau propagation.
Another series of seeding experiments, using a subset of the samples that were identified as low, intermediate or high seeders according to the biosensor assay were conducted. Regardless of treating primary neuron cultures with extracted soluble tau proteins or injecting extracts directly into mouse brains expressing mutated P301S tau, similar relative trends in seeding activity were observed among the samples.
How Tau Determines Seeding Potency
The researchers subjected tau from brain extracts to a barrage of biochemical assays, then cross-referenced the results with seeding activity gleaned from biosensor assays. The results indicated that seeding activity was correlated not with the total amount of tau, but specifically with levels of oligomeric, hyperphosphorylated tau. Compared with intermediate or low seeders, high seeders had an abundance of soluble, high-molecular-weight tau oligomers.
Researchers used mass spectrometry to map the phosphorylation landscape of tau across the samples. Tau doubly phosphorylated on Thr231 and Ser235, and singly phosphorylated on Ser262. This rate of phosphorylation correlated with seeding activity. Neither ptau-181 or ptau-217 — both species of tau that rise in cerebrospinal fluid during the preclinical stages of AD — were significantly tied to seeding activity.
Findings indicated that higher tau seeding activity correlated with a steeper rate of patient decline as measured by the CDR-SOB, and a younger age at symptom onset. The research team was thus able to tie aggregation-prone forms of tau in the postmortem brain to a more rapid course of disease. According to their findings, the most hazardous species are large, soluble tau oligomers, phosphorylated on specific residues.
Prognosis and Treatment Potential
The Massachusetts General team’s findings make it clear that tau’s behavior can be used as a prognostic determinant for AD. Instead of directly measuring seeding activity, researchers could quantify tau oligomers or specific phospho-tau species associated with seeding activity. Targeting these specific forms of tau proteins with therapeutics could be a promising new treatment option for AD patients.
In this study, tau seeding activity accounted for about 25 percent of the clinical heterogeneity among people with typical AD. This suggests that tau antibodies that block tau seeding may inhibit AD’s clinical progression. In further experiments, researchers used a panel of seven antibodies directed against different parts of the tau protein or against specific phospho-residues to deplete tau from brain extracts, then tested the remaining seeding activity. Overall, the experiments indicated that antibodies — most notably AT8 and PHF1 — that impede tau seeding may present a promising treatment option.
QPS is a GLP- and GCP-compliant contract research organization (CRO) delivering the highest grade of discover, preclinical and clinical drug research development services. Since 1995, it has grown from a tiny bioanalysis shop to a full-service CRO with 1,100+ employees in the U.S., Europe and Asia. Today, QPS offers expanded pharmaceutical contract R&D services with special expertise in neuropharmacology, DMPK, toxicology, bioanalysis, translational medicine and clinical development. An award-winning leader focused on bioanalytics and clinical trials, QPS is known for proven quality standards, technical expertise, a flexible approach to research, client satisfaction and turnkey laboratories and facilities. Through continual enhancements in capacities and resources, QPS stands tall in its commitment to delivering superior quality, skilled performance and trusted service to its valued customers. For more information, visit www.qps.com or email [email protected].