WELCOME TO QPS
CUSTOM-BUILT RESEARCH™
A GLOBAL LEADER IN BIOANALYSIS, PRECLINICAL
AND CLINICAL RESEARCH SERVICES
Why QPS?
Partnering with the QPS Preclinical and Clinical teams is easy!
To begin, we set up a client-dedicated team to meet your specific study requirements. For maximum flexibility and efficiency, an experienced Project Manager will have overall responsibility for the conduct of your study and that person will be your single point of contact for the duration of the study.
Agility
Agility
Flexibility
Flexibility
Speed
Speed
Quality
Quality
How can we help you accelerate Pharmaceutical Breakthroughs?
Toxicology, DMPK, Bioanalysis, Gene Therapy, Early and Late Phase Clinical Trials, Recruitment Services and Contract Research Support Services form the backbone of the broad range of QPS service offerings.
Custom-Built Research Service Packages
More than 25 Years of Experience!
With more than 25 years of experience, divisions that specialize in every facet of drug development and a senior leadership team with several hundred years of combined industry experience, QPS is uniquely positioned to be your partner.
Pharmacology
Pharmacology
DMPK Services
DMPK Services
Bioanalysis
Bioanalysis
Assay Finder
Assay Finder
Translational Medicine
Translational Medicine
Regulatory and Medical
Regulatory and Medical
Full-Service Contract Research Organization
QPS offers a comprehensive range of clinical research services. Our support for our pharmaceutical, biotechnology, and CRO partners is unrivaled.
As a preclinical CRO, we have the experience and knowledge required to take a pharmaceutical product through the testing phase to clinical trials in a timely manner. The breadth and depth of our drug development offerings are foremost in the industry and we look forward to discussing how we can support your needs.
Spotlight
QPS Custom-Built Research
Agility. Flexibility. Speed. QPS showcases an animated logo to demonstrate how we embody the unique spirit of the hummingbird.

QPS Assay Finder
The QPS Assay Finder contains details of over 800 assays that QPS has on-hand for your bioanalytical studies. Download the free App from the Apple Store, or from Google Play, or Click here to access the Assay Finder on this website.

QPS Holdings, LLC Sells QPS Neuropharmacology to Scantox
QPS announces an agreement to sell its Neuropharmacology business unit (QPS Neuropharmacology) to Scantox, a leading Nordic pre-clinical, GLP-accredited CRO.

French Research Tax Credit
QPS is delighted to announce that we have received accreditation for the French Research Tax Credit (CIR) for 2023, 2024, and 2025.

Galecto Head Praises QPS
QPS is proud to have received high praise from Galecto for our recent Phase I Bioavailability and Food Effect study product.
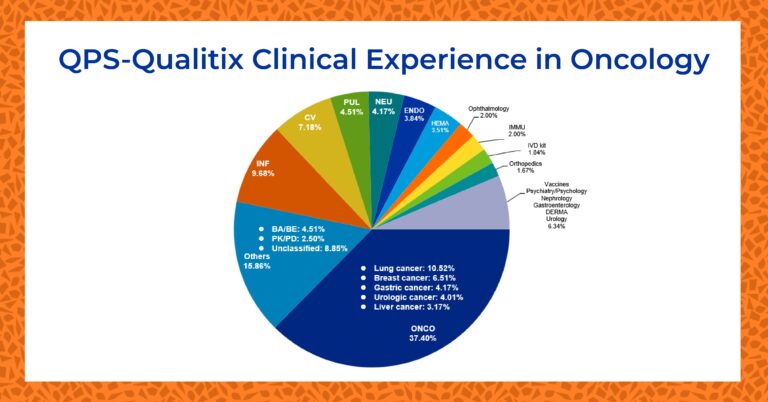
QPS Phase I Oncology Experience
Oncology studies make up the greatest percentage of clinical trials completed at QPS Qualitix. Since 2015, more than 40% of all studies conducted have been oncology trials.