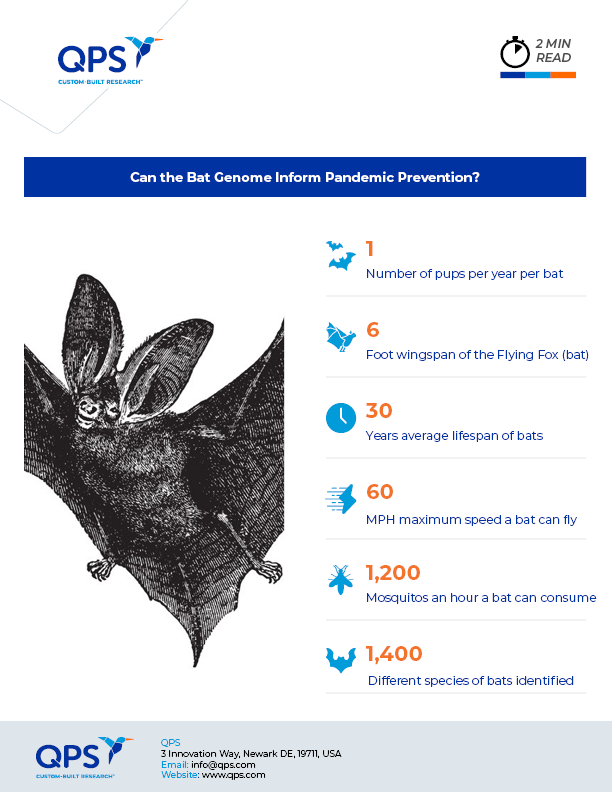
Research on the link between bats and human health has gained significant attention in recent years due to the outbreaks of a number of zoonotic diseases, including SARS-CoV-2, or COVID-19. The ongoing COVID-19 pandemic has only further highlighted the need for a better understanding of the relationship between wildlife — especially bats — and human health. And with new research focused on understanding the relationship between coronaviruses and bats, some scientists believe that studying the genetic information contained in the bat immune system could hold the key to developing new treatments for a range of human diseases and help to predict and prevent future pandemics.
The Link Between the Bat Genome and Humans
The link between bats and human diseases predates the current COVID-19 pandemic. While bats are not the only mammals that can spread diseases to humans, bats have long been linked to a number of serious infectious diseases, particularly coronaviruses. These include SARS-CoV-2, severe acute respiratory syndrome-associated coronavirus (SARS-CoV), and Middle Eastern respiratory syndrome-associated coronavirus (MERS-CoV), three serious coronaviruses believed to have originated in bats. And as bats have been at the forefront of discussions surrounding the origin of the COVID-19 pandemic, there has been a renewed focus on understanding the link between bats and coronaviruses.
New research recently published in the Proceedings of the Royal Society B journal focused on just that, examining the evolutionary pressures that the bat immune system faces in response to coronaviruses and other diseases that can spread to humans.
Genetic Pressure
The team of researchers, led by Hannah Frank, an assistant professor in the ecology and evolutionary biology department at Tulane University, focused specifically on investigating two host proteins: the angiotensin-converting enzyme 2 (ACE2) and dipeptidyl-peptidase 4 (DPP4). ACE2 acts as a receptor for the virus that causes both COVID-19 and SARS-CoV, while DPP4 is the receptor for the virus which causes MERS-CoV.
For their research, the team analyzed the largest bat genetic datasets to date, generating new ACE2 and DPP4 sequences from 55 bat species representing five families and 37 genera.
Frank and her team found that in bats, both the ACE2 and DPP4 genes are under strong selection pressure, notably more so than in other mammals.
Importantly, the team also learned that mammalian groups demonstrate a range of variation in their similarity to humans in residues that SARS-CoV, SARS-CoV-2, and MERS-CoV. As Frank explains, that “increased similarity to humans in binding residues is broadly predictive of susceptibility to SARS-CoV-2.”
Further Research
Frank’s research confirmed that the bat immune system is under more pressure from coronaviruses than other mammalian immune systems. As a result, bats are indeed a significant source of a number of coronaviruses. But Frank clarifies that bats remain an important and necessary part of our ecosystem — in the United States alone, they save billions of dollars annually through pest control, plant pollination, and seed spreading — and that this research does not mean that humans should start to fear bats.
Instead, Frank says, the study highlights the importance of ongoing research focused on the relationship between mammals and coronaviruses, particularly when it comes to learning how host immune systems have evolved in response to these viruses. The better researchers understand the interaction between coronaviruses and a diverse range of mammalian hosts, the better prepared the scientific community will be to identify probable sources of zoonotic infection, secondary reservoir species, and groups of animals that may be susceptible to infection. The better we understand, for example, how the bat immune system has evolved along with coronaviruses, the better we may be able to use this data to inform conservation and public health efforts.
The study emphasizes the importance of studying natural hosts such as bats to better understand the genetic mechanisms of virus-host interactions. And as Frank said, “It also highlights broad patterns in susceptibility that may prove useful for managing this and future pandemics.”
QPS is a GLP- and GCP-compliant contract research organization (CRO) delivering the highest grade of discovery, preclinical and clinical drug research development services. Since 1995, it has grown from a tiny bioanalysis shop to a full-service CRO with 1,200+ employees in the U.S., Europe and Asia. Today, QPS offers expanded pharmaceutical contract R&D services with special expertise in neuropharmacology, DMPK, toxicology, bioanalysis, translational medicine and clinical development. An award-winning leader focused on bioanalytics and clinical trials, QPS is known for proven quality standards, technical expertise, a flexible approach to research, client satisfaction and turnkey laboratories and facilities. Through continual enhancements in capacities and resources, QPS stands tall in its commitment to delivering superior quality, skilled performance and trusted service to its valued customers. For more information, visit www.qps.com or email [email protected].