Accelerate Your Drug Development with QPS Real-World Evidence (RWE) Services

Harnessing Real-World Data for Innovative Insights
At QPS, we leverage Real-World Evidence (RWE) to transform drug development and enhance patient outcomes. Our comprehensive RWE services utilize routinely collected patient health data, known as Real-World Data (RWD), to deliver insights beyond traditional clinical trials. This data-driven approach helps fill evidence gaps, supports more inclusive research, reduces reliance on animal models, and accelerates access to innovative therapies.
Why QPS for Real-World Studies?
Innovation
Innovation
Data Resources
Data Resources
Global Operations
Global Operations
Therapeutic Experts
Therapeutic Experts
Integrative Specialty Data
Integrative Specialty Data
Powerful Persuasive Insights
Powerful Persuasive Insights
Advanced Algorithms
Advanced Algorithms
World Class Regulatory Expertise
World Class Expertise
GDPR and HIPAA-Compliant
GDPR and HIPAA-Compliant
Specialized Product Areas
At QPS, we combine therapeutic expertise with experience in optimizing and executing RWE studies for specialized product areas and patient populations to uncover your product’s impact on patient outcomes.
We have experience across more than 15 major therapeutic areas including:
- Cardiovascular
- Central Nervous System
- Dermatology
- Endocrinology/Diabetes
- Gastroenterology
- Immunology
- Infectious Disease
- Obesity
- Oncology
- Ophthalmology
- Pulmonology
- Rare Diseases
- Rheumatology
- Vaccines
- Women’s Health
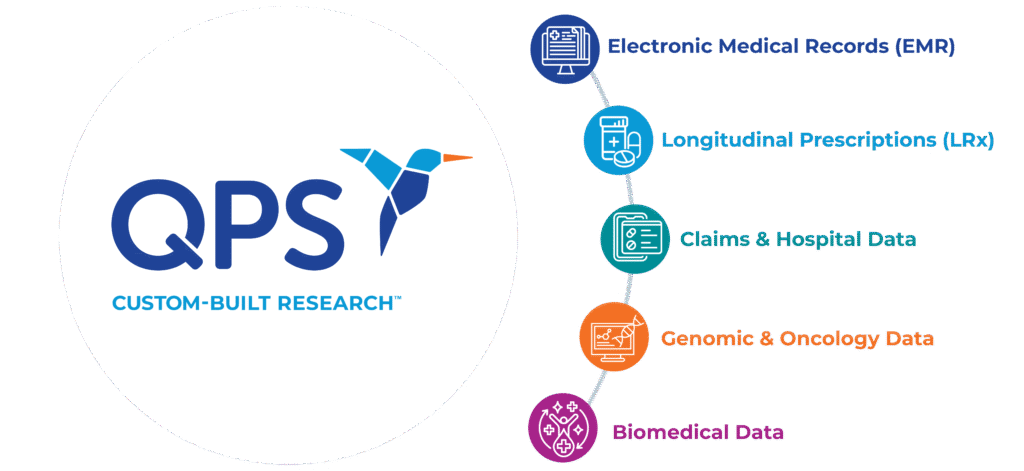
Types of QPS Data Available
Data access and management processes have evolved given the fast pace evolution of big data transformation technology, Artificial Intelligence, and increased importance of Real-World Data. QPS benefits from strong expertise in managing proprietary data sets and has invested in Artificial Intelligence platforms to collect or enable access to data to be cleaned, curated, bridged, harmonized, and/or linked. The additional ability to de-identify data for compliant use enables us to generate evidence that leads to insights to support your next Real World Evidence project.
Why Choose QPS?
- Global Reach and Infrastructure: Our global infrastructure includes clinical research units and over 700 partner sites across the US, Europe, Asia, Australia, and India, allowing us to provide comprehensive support at every stage of the product lifecycle.
- Leveraging the Power of Artificial Intelligence (AI): QPS has partnered with Ryght AI, in order to leverage Ryght’s cutting-edge AI technology. Coupling QPS’s experience and global presence in clinical trials with AI technology sets QPS up to generate real world evidence clinical insights in record time.
- Expertise Across Therapeutic Areas: Our therapeutic agnosticism allows for data-driven strategies across a diverse range of indications, including oncology, CNS disorders, dermatology, vaccines, and rare diseases.
- Multidisciplinary Team: Our client-first model is underpinned by a multidisciplinary team of data scientists, biostatisticians, medical writers, and pharmacovigilance experts, all working seamlessly under one umbrella to deliver comprehensive project support.
- Lifecycle Support: From early-stage research to post-marketing surveillance and label expansions, QPS assembles the right data, analytical tools, and expertise to meet evolving project demands effectively.
- Global Reach and Infrastructure: Our global infrastructure includes clinical research units and over 700 partner sites across the US, Europe, Asia, Australia, and India, allowing us to provide comprehensive support at every stage of the product lifecycle.
- Leveraging the Power of Artificial Intelligence (AI): QPS has partnered with Ryght AI, in order to leverage Ryght’s cutting-edge AI technology. Coupling QPS’s experience and global presence in clinical trials with AI technology sets QPS up to generate real world evidence clinical insights in record time.
- Expertise Across Therapeutic Areas: Our therapeutic agnosticism allows for data-driven strategies across a diverse range of indications, including oncology, CNS disorders, dermatology, vaccines, and rare diseases.
- Multidisciplinary Team: Our client-first model is underpinned by a multidisciplinary team of data scientists, biostatisticians, medical writers, and pharmacovigilance experts, all working seamlessly under one umbrella to deliver comprehensive project support.
- Lifecycle Support: From early-stage research to post-marketing surveillance and label expansions, QPS assembles the right data, analytical tools, and expertise to meet evolving project demands effectively.

Contact Us
Ready to accelerate your drug development with QPS RWE services?
Contact us today to discuss how we can help you apply RWD/RWE integration for more efficient product development.
Visit: http://www.qps.com/
Set Up a Meeting: http://www.qps.com/contact
Email: info@qps.com





