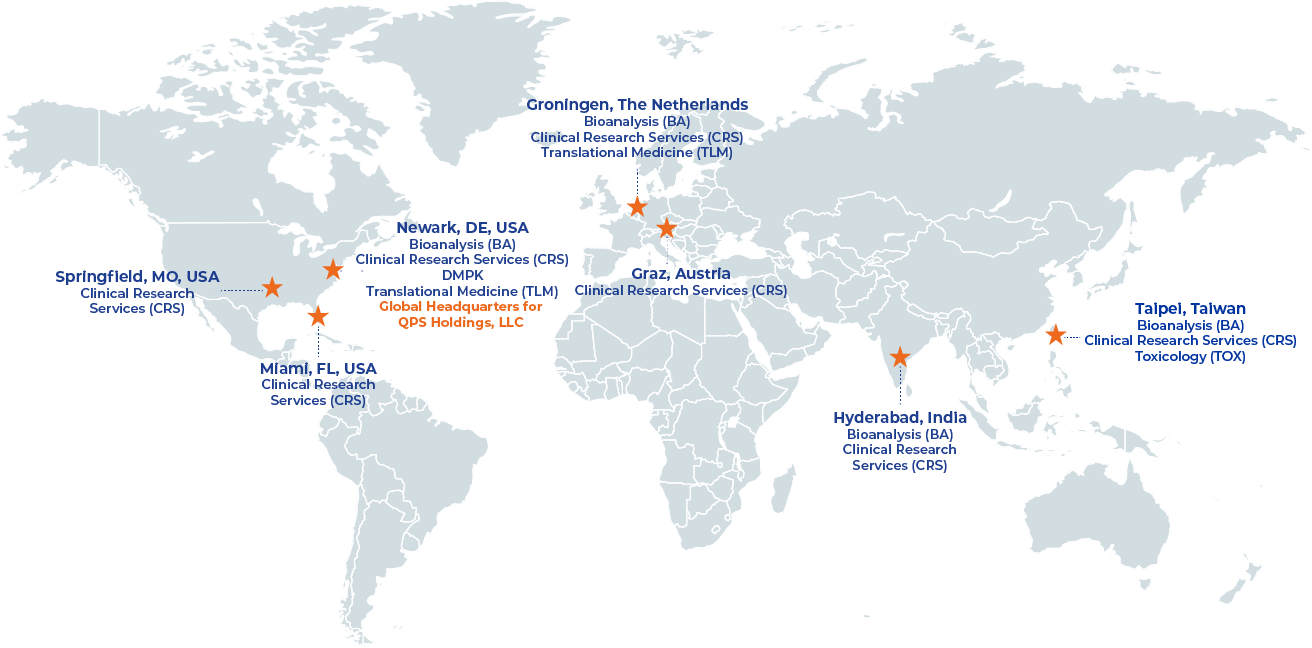
Overview
With its site management & monitoring teams operating from 30 locations on three continents (Asia/Pacific, USA and Europe), QPS has become a new strong player in the space of late phase clinical research services.
Widely recognized for achieving high-quality study data and high patient enrollment rates, QPS’ site management & monitoring teams bring years of experience catering to the unique needs of virtual, small, mid-size and large pharmaceutical and biotechnology firms.
We conduct and manage clinical studies in accordance with International Conference on Harmonization-Good Clinical Practice (ICH-GCP) standards and all applicable regulatory requirements to pharmaceutical and scientific clients.
Why Should QPS Be Your First Choice To Conduct Your Phase II-IV Studies?
Professional and Experienced Staff
With QPS you get immediate access to its Global Clinical Development Teams composed of well-trained Clinical Research Associates, Clinical Research Coordinators, Clinical Project Managers, Regulatory Affairs Staff, Data Managers, Biostatisticians, Clinical Pharmacokineticists, Medical Writers, Quality Control Staff, Safety Reporters and Clinical Research Trainers who are capable of meticulously managing the conduct and taking on the overall responsibility for your clinical trials.
A Full Service CRO
QPS can assist you with the entire late stage drug development process:
- Program Management
- Project Management
- Site Management
- Site Monitoring
- Data Management
- Global Flexibility
- Global Capacity
High Quality Data
- Manage increasing amounts of clinical data from multiple sources and systems
Costumer Focus
QPS focuses on the needs of each client and works to make sure that those needs are met. We strive to make certain all studies are recruited fully and completed on time. We also strive to ensure optimal communications so you always have complete visibility into your project’s status and can rest assured that your deadlines will be met and your budgets will not be exceeded. At QPS, we measure our success by your success.
Preferred Provider Relationship
Improve the efficiency and effectiveness of your drug development efforts and qualify for volume discounts by embracing QPS as your preferred provider for your specific drug development sourcing needs. You will be assigned a dedicated program manager and enjoy maximum benefit of conducting different types of studies at distinct global sites in order to manage your overall drug development costs.
Robust Investigator Network and Therapy Area Expertise
- Cardiovascular Disorders
- CNS Disorders
- Dermatology
- Diabetes & Metabolic Disorders
- Neurology
- Gynaecology & Urology
- Infectious Diseases
- Oncology
- Pain/Inflammation/Musculoskeletal
- Respiratory Disorders