QPS Missouri
QPS Campus
1820 W. Mount Vernon St., Springfield, MO 65802
QPS Screening and Recruitment Center
2025 W. Sunshine St., L-109, Springfield, MO 65807
Click here for a comprehensive look at the QPS Missouri site located in Springfield, Missouri.
Years Serving Clients
0
Clinical Trials Beds
0
Successful FDA Inspections
0
Clinical Studies Completed
0
QPS Missouri Videos
Core Competencies
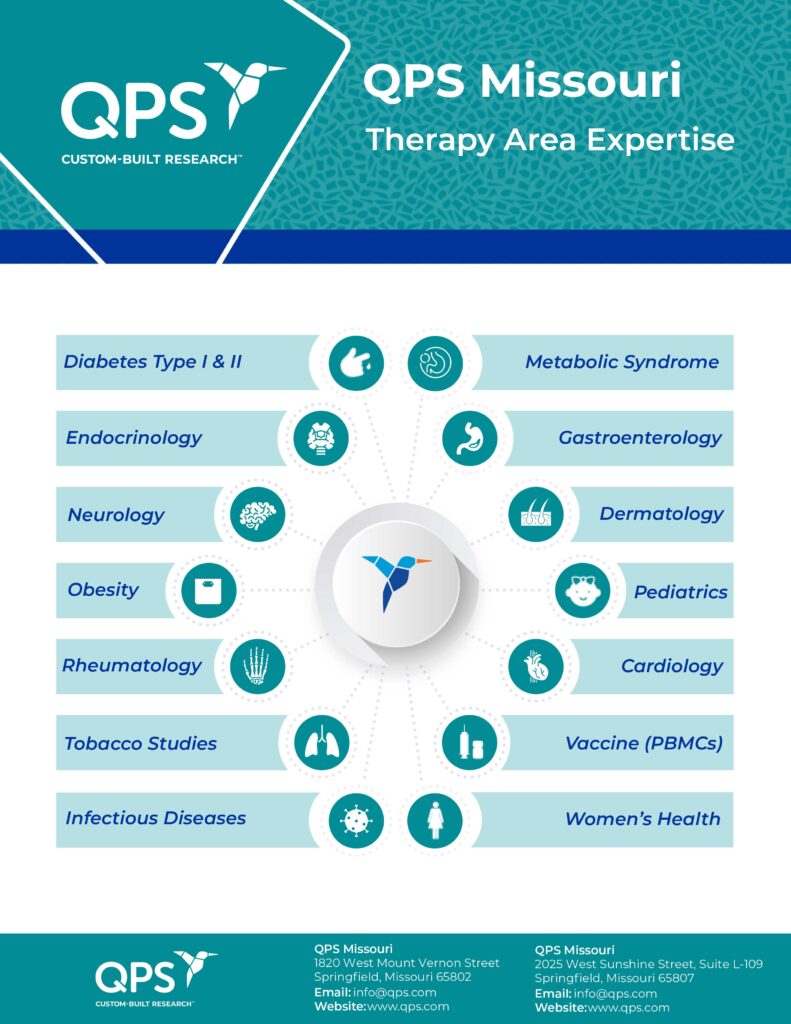
- Phase I and II Clinical Studies
- Smoking Cessation
- Vaccinnes
- Women's Health
- Bioavailability
- Bioequivalence
- Nutrition
- Data management
Facility
- 240 beds across 5 updated clinical units designed for open-concept layout.
- 24-hour security systems and automatic natural gas-powered generators for power backup.
- Secure on-site archive/document storage with FM-200 gas fire suppression.
- Laminar flow hoods; -70˚C and -20˚C freezers.
- 14 micro-, table-top, and floor centrifuges, and high-speed micro-centrifuges.
- National Institute of Standards and Technology (NIST) calibrated temperature recording devices in refrigerators and freezers.
- Secure pharmacy and retention with DEA-approved safe for Schedule I-IV drugs.
- Local clinical lab provides most results within 24 hours, 7 days a week.
People
- Highly experienced principal investigators (PIs) with as much as 20+ years’ experience in clinical research.
- Leadership team includes technology and CRO services experts with 20+ years of experience.
- Average management team experience includes 10+ years in CRO.
- Dedicated Institutional Review Board (IRB) conducts reviews in accordance with pertinent authorities, including ICH E6: Good Clinical Practice Consolidated Guidance; US FDA (21 CFR Parts 50 and 56); and the US Department of Health.
Accreditation
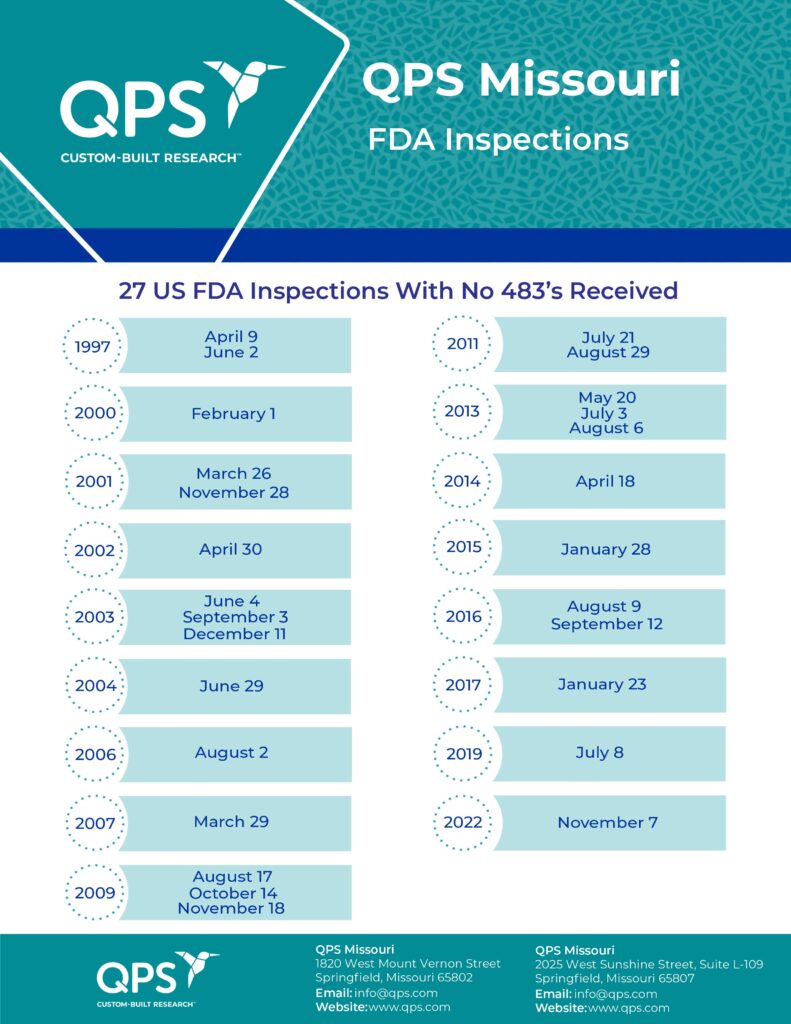
- 21 successful clinical site US FDA inspections.
- No 483 findings in the past 4 years.
Experience
- Over 1,500 Phase I-IV clinical research studies completed since 1994.
- 100% source and entry quality control.
QPS Missouri
- QPS Missouri offers a broad range of services to support drug development research, and to design and conduct Phase I clinical studies.
- Established in 1994, QPS Missouri is located on a 5-acre, independently-owned campus, with 80+ employees.
QPS Missouri Clinical Site Overview
The QPS Clinical Research site in Springfield, MO, USA is an impressive facility in a great location. If you are a sponsor looking for a site, or a potential study subject looking to participate in a clinical trial, this interactive resource provides a closer look at the facility and surrounding area.