Obesity Clinical Trials: Advancing Weight Loss Research with QPS
The development of obesity drugs and their clinical trials represents a dynamic and rapidly evolving field, aiming to address the global health challenge of obesity. As of 2022, global statistics indicate that over 890 million adults are affected by obesity. This means that approximately 1 in 8 people worldwide are living with obesity. The pursuit of potent obesity treatments is gaining momentum, with projections estimating the market’s worth to reach $37.1 billion by 2031.
Obesity Clinical Trials are crucial in the process of drug development, evaluating not just the safety and effectiveness of novel medications and approved medications for new indications but also their long-term effects on patient well-being and quality of life. Attention has centered on glucagon-like peptide-1 receptor agonists (GLP-1s)—originally developed for type 2 diabetes—yet now emerging as highly effective options in managing obesity. As more trials are conducted globally, researchers aim to meet the unmet need for a wider range of treatments to cater to the diverse patient population affected by obesity.
📌 Read the QPS blog posts discussing obesity here.
QPS’s Role in GLP-1 Trials & Obesity Drug Development
Advancing GLP-1 Clinical Trials for Obesity Treatments
QPS plays a critical role in the development of GLP-1 receptor agonists and other emerging obesity treatments by offering comprehensive Phase I-IV clinical trial services. With the growing demand for weight loss drugs, including semaglutide, tirzepatide, and other next-generation GLP-1 therapies, QPS provides the expertise necessary to ensure regulatory compliance, patient safety, and clinical efficacy assessments with a very low study subject drop out rate.
Our dedicated research teams work closely with pharmaceutical and biotech companies to optimize study protocols, streamline regulatory submissions, and ensure that trials progress efficiently from early-phase first in man clinical trials to late-stage global multi-site registration trials.
Expertise in Obesity Drug Development
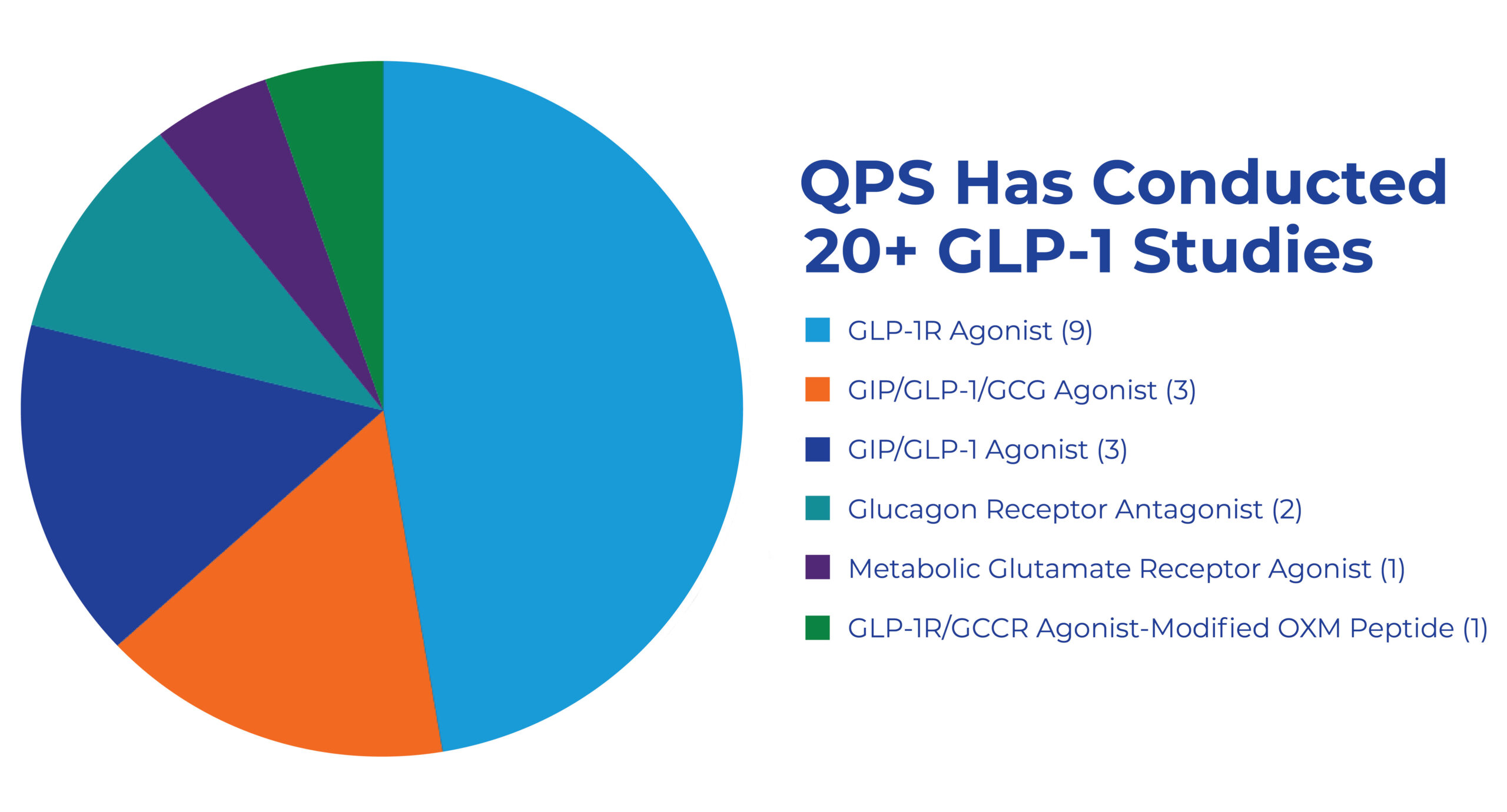
QPS has conducted over 13 successful obesity trials since 2015, including multiple GLP-1, GIP, and GIP/GLP-1 studies.
- Comprehensive trial design tailored to obesity and metabolic disorder studies.
- Pharmacokinetics (PK) and Pharmacodynamics (PD) modeling to evaluate drug absorption, metabolism, and efficacy.
- Advanced biomarker analysis to assess treatment effectiveness.
- Safety and tolerability monitoring to track adverse events and long-term outcomes.
Obesity Clinical Trials are Complex
Clinical trials for obesity face significant challenges related to maintaining trial integrity and ensuring treatment effectiveness. At QPS, these challenges are overcome through careful trial design, rigorous monitoring, and patient education. By addressing these complexities, QPS is able to maintain patient safety, uphold the integrity of the trials, and gather reliable data that supported the effectiveness of the treatments.
Our experience has paved the way for successfully managing obesity drug development programs from Phase I to global Phase III registration trials in patients.
Successful Trials:
Over 13 successful obesity trials since 2015, including multiple GLP-1, GIP, and GLP-1/GIP studies.
Global Research:
Access to a diverse patient population through our extensive network of clinical sites. By leveraging our global research network, QPS helps sponsors accelerate drug development and bring new obesity treatments to market faster.
Phase I Expertise:
Our clinics are highly experienced in complex FIH SAD/MAD trials, supported by a large healthy volunteer database. Studies are conducted seamlessly, using alternates to ensure timelines are met. Principal investigators are full-time employees, accessible to both patients and sponsors for questions and concerns, with a very hands on approach.
Subject Matter Experts:
QPS’s full-time staff includes physicians with both early and late phase clinical trial experience. They provide support before the trial starts with protocol eligibility questions and during the trial to resolve adverse events and patient complexities. Additionally, QPS has clinical pharmacology experts to support proper design and to answer pharmacokinetic (PK) questions.
Proper Site Selection:
Selecting the right number of sites for obesity trials from the start ensures timely enrollment and study cohort success. Based on our experience, rapid enrollment in obesity trials can often be done at one site. QPS has experience rapidly enrolling large numbers of study subjects for obesity trials in just one clinical trial site.
Pre-identification of Obesity Subjects:
Successful cohorts rely on more than databases alone. Social media (Instagram and Facebook), radio, well-targeted advertisements, and recruitment at local events can all help identify new subjects. Ideally, subject identification for the patient cohort should begin while completing the healthy volunteer portion of the trial, ensuring patients are available once the patient portion starts.
Full Service Global Clinical Research Services:
QPS can manage your Phase I – IV clinical trials from start to finish. With regulatory and medical affairs support, sample analysis, clinical trial kit production, data management, project management, site selection, and monitoring, QPS can ensure your trial is completed successfully.
Partner with QPS for Obesity Clinical Trials
What classes of drugs are currently being used in obesity?
Each class of drugs works through a different mechanism to aid in weight loss. In some cases, the drugs listed below have been surpassed by other, newer, more effective and/or safer drugs. The most effective of the available drugs are GLP-1s, which were originally formulated and approved for Type 2 diabetes and were “accidentally” found to be effective weight loss drugs. Read the QPS blog post on weight loss injectables here.
- Pancreatic Lipase Inhibitors that reduce the adsorption of dietary fat
> Orlistat
- Stimulants that suppress appetite
> Phentermine
> Diethylpropion
> Benzphetamine
> Phendimetrazine
- Combinations that control appetite and increase satiety
> Phentermine/Topiramate
> Bupropion/Naltrexone
- MC4R agonists indicated for rare genetic disorders of obesity
> Setmelanotide
- Glucagon-like peptide-1 receptors (GLP-1s) that help increase the feelings of fullness. Only Wegovy and Zepbound have been approved for weight loss by the FDA. The other GLP-1s listed below are approved for Type 2 diabetes, but many are being studied for an obesity indication.
> Semaglutide
> Tirzepatide
> Dualglutide
> Exenatide
> Exenatide
> Liraglutide
> Semaglutide
The introduction of the GLP-1 class of drugs has transformed the weight loss market. These medications routinely deliver 10 – 20% reductions in overall body weight, which is far greater than the previously available options. Unfortunately, since weight loss is often classified as a lifestyle disease, most insurance companies are not covering these new therapies. In addition, the burden of obesity falls disproportionately on the Medicaid population, and Medicaid is not covering these drugs either.
Moving forward, these drugs are being considered for use in additional indications, such as cardiovascular disease, metabolic dysfunction-associated steatohepatitis (MASH) and addiction, which may cause insurers to take a second look at the overall value they deliver to the health of the population. All weight loss medications are typically prescribed alongside lifestyle modifications, such as diet and exercise, and are intended for long-term use as part of a comprehensive weight management plan overseen by a healthcare professional.
The field of obesity treatment is in the midst of a gold rush, with at least 124 medicines currently in clinical trials: Phase 1 (61), Phase 2 (42), Phase 3 (8), and 8 are on the market. Many of these potential new therapies are GLP-1’s, although there are other mechanisms of action being studied too, including cell and gene therapies. Learn more about cell and gene therapy here.
How is cell and gene therapy advancing obesity treatment?
Recent research has uncovered new methods to potentially harness “good fat” cells to combat obesity and control diabetes. For instance, one study revealed structural details of uncoupling protein 1 (UCP1) in brown adipose tissue. This protein allows fat tissue to burn off calories as heat, presenting a potentially viable route for shedding weight via the process of thermogenesis.
Moreover, researchers are actively exploring AMPK-targeting peptides for their potential to enhance mitochondrial function and regulate elevated blood glucose levels, prevalent in diabetes and obesity. Demonstrating efficacy in early-stage studies with mice and human cells, these peptides may become integral to forthcoming cell therapy treatments.
These advancements indicate that cell therapy could offer a new dimension to obesity treatment, targeting the condition at a cellular level and providing a more comprehensive approach to managing this complex health issue.
Although these developments are encouraging, it’s crucial to recognize that cell therapy for obesity remains in a research phase, necessitating many years of further investigation before it can be utilized as a treatment option. Learn more about cell therapy here.
Gene-editing methods such as CRISPR are being investigated for optimizing metabolic processes and body weight management, potentially elevating quality-of-life and health outcomes for individuals with obesity and/or diabetes. Additionally, research is actively being conducted on ex vivo gene therapy for obesity, which involves harvesting cells and altering cells externally before reintroducing them into the body to address the underlying disease. Learn more about gene therapy here.
A major benefit of gene therapy compared to conventional treatments is its ability to significantly lower body weight. While the new GLP-1 anti-obesity drugs typically target a 10% – 20% reduction, gene editing with CRISPR–Cas9 has achieved remarkable results, consistently reducing body weight by up to 20% in certain studies.
Although gene therapy for obesity is in its research phase, its potential to deliver more substantial and enduring weight management solutions is promising. With ongoing advancements, gene therapy may soon become an essential instrument in addressing obesity, providing new hope for individuals grappling with weight management.
While there are no cell or gene therapies that have been approved for the treatment of obesity, several promising therapies are being studied in clinical trials. The focus has been on understanding the genetic factors that contribute to obesity and developing therapies that can target these factors to provide a more effective and long-lasting solution to weight management. There have been significant advancements in cell therapies and gene editing technologies like CRISPR, designed to convert white fat cells into brown-fat cells, these are not yet available as approved treatments for obesity.
The development of an obesity drug, from initial discovery through regulatory approval, typically takes 10-15 years1. This process involves a series of phases, including preclinical research, clinical trials, and finally, review and approval by regulatory bodies such as the FDA. However, it’s important to note that this is an average estimate, and the actual time can vary depending on various factors such as the complexity of the drug, the results of clinical trials, and the speed of regulatory review. Learn more about how QPS can help manage your global regulatory affairs pathway here.





