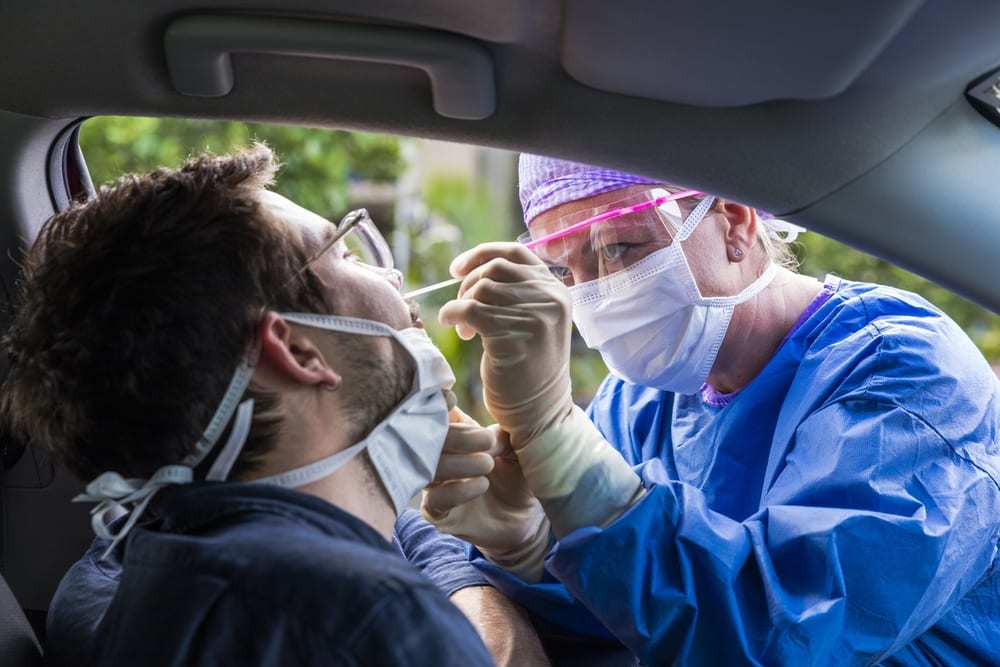
Antigen Testing
Part I of this series on testing for the novel coronavirus covered the real-time reverse transcription polymerase chain reaction (rRT-PCR) assay, which is administered via nasal swab and can diagnose an active infection. Part II covered serology testing, which scans blood samples for antibodies to the virus and can indicate a previous infection — and potential immunity.
Our third installment in this series focuses on antigen testing. This type of testing detects antigens via a nasal swab, indicating an active coronavirus infection. It does not require any special training or equipment (except for the nasal swabs, which currently have supply chain issues), can give a result in 30 minutes, can be manufactured at scale, and will cost only about USD $10. It is not, however, without its own set of challenges.
Antigen Testing: The Process
An antigen is a part of a pathogen that elicits an immune response, causing the immune system to create antibodies. Antigens can be part of any substance that comes from outside the body, including viruses or bacteria.
An antigen test searches for fragments of viral surface proteins as a marker for active infection. In the case of the novel coronavirus, these proteins are from the virus’s surface spikes and are large enough to study on their own. This makes the antigen test easier to administer than rRT-PCR tests, in which the tiny RNA fragments must be copied thousands of times in order to be detectable.
An antigen test begins with a nasal swab, similar to the PCR test. The swab is then put into a solution, and the solution is exposed to one end of a paper strip. The strip contains artificial antibodies that are designed to bind to the coronavirus antigens. As the solution moves up the paper strip, the antigens present will bind to the artificial antibodies and give a visual readout.
The coronavirus antigen test was created from the same basic platform that was used for the Zika virus and dengue fever virus antigen tests — both of which are 90%–95% accurate. Researchers predict that the same level of accuracy can be reached for the coronavirus antigen test.
Companies such as OraSure and E25Bio both have antigen tests in development and expect to eventually be able to produce millions of tests. They are also working on accompanying apps that will securely collect anonymous data, helping epidemiologists track the size and spread of the pandemic.
Challenges with Antigen Testing
One of the major challenges with coronavirus antigen tests is that they are not easy to develop. Four months into the pandemic, scientists are finally beginning to understand the biology and structure of the coronavirus well enough to create a reliable antigen test. The key to success is discovering which proteins to look for.
But even more problematic is the possibility that an antigen test will not work for the coronavirus. Antigen tests work very well for bacterial diseases—such as streptococcal pharyngitis — and some viral diseases, but respiratory viruses behave much differently. For example, the influenza virus antigen test has about 70%–80% detection sensitivity — but only in children. This is because children carry a much higher viral load; with adults, the influenza test is about 50% accurate. This lack of test sensitivity is typical for respiratory viruses.
Additionally, the presence of the virus in the nasal cavity varies from person to person. Self-swabbing, although it does not require any special training, is invasive and uncomfortable, which may present a barrier to test accuracy.
Both E25Bio and OraSure have manufactured antigen tests for other diseases, but not for respiratory viruses. Antigen testing developers’ estimates of 90% accuracy are based on lab-generated samples; they have not yet tested patient samples, which could be less accurate.

RT-PCR and Serology Testing vs. Antigen Testing
Some scientists posit that we will need to be testing upwards of 20 million people per day in order to safely relax stay-at-home orders; the United States is currently performing about 200,000 tests per day. According to Deborah Birx, the response coordinator for the White House Coronavirus Task Force, “There will never be the ability on a [PCR] test to do 300 million tests a day or to test everybody before they go to work or to school. But there might be with the antigen test.”
Both PCR and serology testing have been plagued by a lack of nasal swabs, testing reagents, and other supplies. Lee Gehrke, a professor at MIT and Harvard Medical School, says that the nature of COVID-19 is such that a patient could be negative one day and positive the next, which makes follow-up testing imperative; but a PCR test can take hours to run and as long as a week to come back, making effective follow-up testing nearly impossible.
Antigen testing can identify the presence of coronavirus antigens in just a few minutes — with no specialized equipment or trained personnel. Thus, antigen testing would be easy to scale up, both in the home and at the point of care. This type of test could function well for the necessary frequent follow-up testing that guarantees a patient is virus-free. The fact that antigen testing can give quick yes-or-no results is especially valuable for settings such as hospitals and nursing homes, as well as in determining whether a healthcare worker can return to work.
Clinicians, researchers, and public health officials recognize that antigen testing will not replace rRT-PCR testing as the gold standard for active infection detection. But the potential for antigen testing to work alongside both PCR and serology testing is promising — potentially breaking up testing bottlenecks and helping the United States get to the 20 million tests per day that it needs to safely return to everyday life.
Are you looking for a CRO to assist with your preclinical or clinical drug development related to the novel coronavirus or COVID-19? QPS has CLIA-certified and GLP-compliant laboratories ready to fast-track your novel coronavirus and COVID-19 RT-qPCR/QPCR and Serological Assays and vaccine development programs. Since 1995, QPS has provided discovery, preclinical, and clinical drug development services. An award-winning leader focused on bioanalytics and clinical trials, QPS is known for proven quality standards, technical expertise, a flexible approach to research, client satisfaction, and turnkey laboratories and facilities. For more information, visit www.qps.com/coronavirus or email covid19study@www.qps.com.







