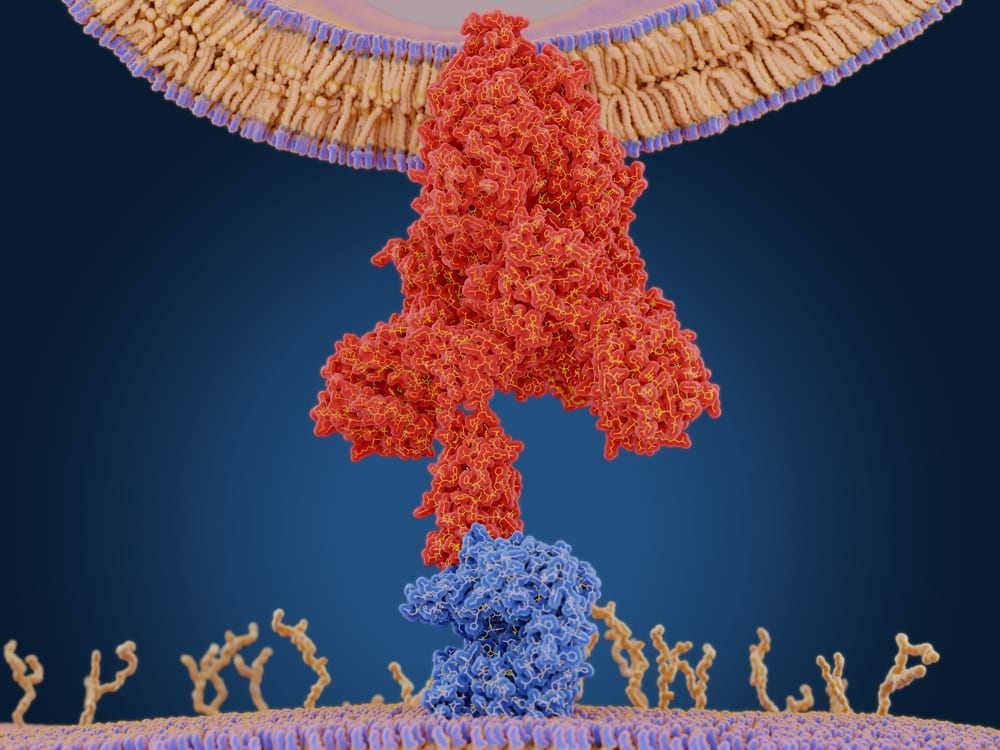
As the COVID-19 pandemic spreads throughout the world, the scientific community learns more about the SARS-CoV-2 virus and plans to use that information to develop new prevention and treatment options. Much of the research focuses on how SARS-CoV-2 enters cells to infect people, and why this virus is more adept at infection than its predecessors. We will be exploring this topic in-depth as a three-part series, starting with the spike protein on the surface of the virus and its role in the infection rate.
A New Spike Protein Structure
A recent article in Nature reviewed research on the virus since its emergence in late 2019, and a large part of this research focused on the structure and function of the spike protein. Researchers have known that the spike protein receptor binding domain (RBD) attaches to the ACE2 receptor on human throat and lung cells. The earlier coronavirus, SARS-CoV, also bound to the ACE2 receptor, but the SARS-CoV-2 binding strength was found to be two to four times stronger than the earlier virus.
Every SARS-CoV-2 viral particle has about 24 to 40 spike proteins on its surface. These spikes enable the virus to fuse with and ultimately enter human cells. Researchers at the Max Planck Institute in Frankfurt, Germany, discovered last year that the SARS-CoV-2 spikes are flexible, with three hinges — this is unusual, as spike proteins found on other viruses typically have a rigid structure. The flexibility allows the SARS-CoV-2 spike to bend and rotate, making it easier to find the ideal place to bond to the surface of human cells.
Like other viruses, the SARS-CoV-2 surface is covered with a sugary coat of glycans, which help hide infectious spike proteins from the host organism’s immune system. In 2020, a team of computational biophysicists at the University of California, San Diego, published a detailed molecular map of the glycan surface. After posting the graphic on social media, a reader added a comment asking about the uncoated loop at the top of the structure. A structural biologist at the University of Texas posted a reply, identifying it as a loop of protein that was the SARS-CoV-2 spike protein’s RBD. Computer models showed that when the RBD rose above the glycan cover, two glycan molecules locked the RBD into place, allowing it to easily bind to human cells. Nearly all SARS-CoV-2 variants also contain the spike mutation called D614G (which substitutes an aspartic acid for a glycine amino acid), making the newest coronavirus more infectious than previous strains, such as those that caused SARS or MERS.
Variant Changes Enhance Infections
Subsequent variants of the SARS-CoV-2 have developed mutations located in the “top” S1 (subunit one) of the spike protein, which contains the RBD. The Nature article points out that the Alpha variant has 10 mutations in the genetic sequence for the RBD, making it easier to stay in its infectious “up” position. The Delta variant also had mutations in the S1, three of which appear to improve the ability of the viral RBD to bind to ACE2 receptors and bypass the human immune system. Compared to past variants, Delta has a shorter incubation period, down from six to four days, and one infected individual will likely infect six people, which doubles previous rates of infection. Scientists believe these changes can be attributed to the mutations in Delta’s S1.
The spike protein mutations are just part of how the SARS-CoV-2 virus became highly infectious. We will continue this exploration in the future blogs in this series, reviewing the interaction of viral and cell membranes, the takeover of host cell metabolism, and a deeper dive into how mutations contribute to the infection rate for Delta and other variants.
Did you enjoy this blog post? Check out our other blog posts as well as related topics on our Webinar page.
QPS has CLIA-certified and GLP-compliant laboratories ready to fast-track your novel coronavirus and COVID-19 RT-qPCR/QPCR and Serological Assays and vaccine development programs. Since 1995, QPS has provided discovery, preclinical, and clinical drug development services. An award-winning leader focused on bioanalytics and clinical trials, QPS is known for proven quality standards, technical expertise, a flexible approach to research, client satisfaction, and turnkey laboratories and facilities. For more information, visit https://www.qps.com/coronavirus/ or email info@qps.com.