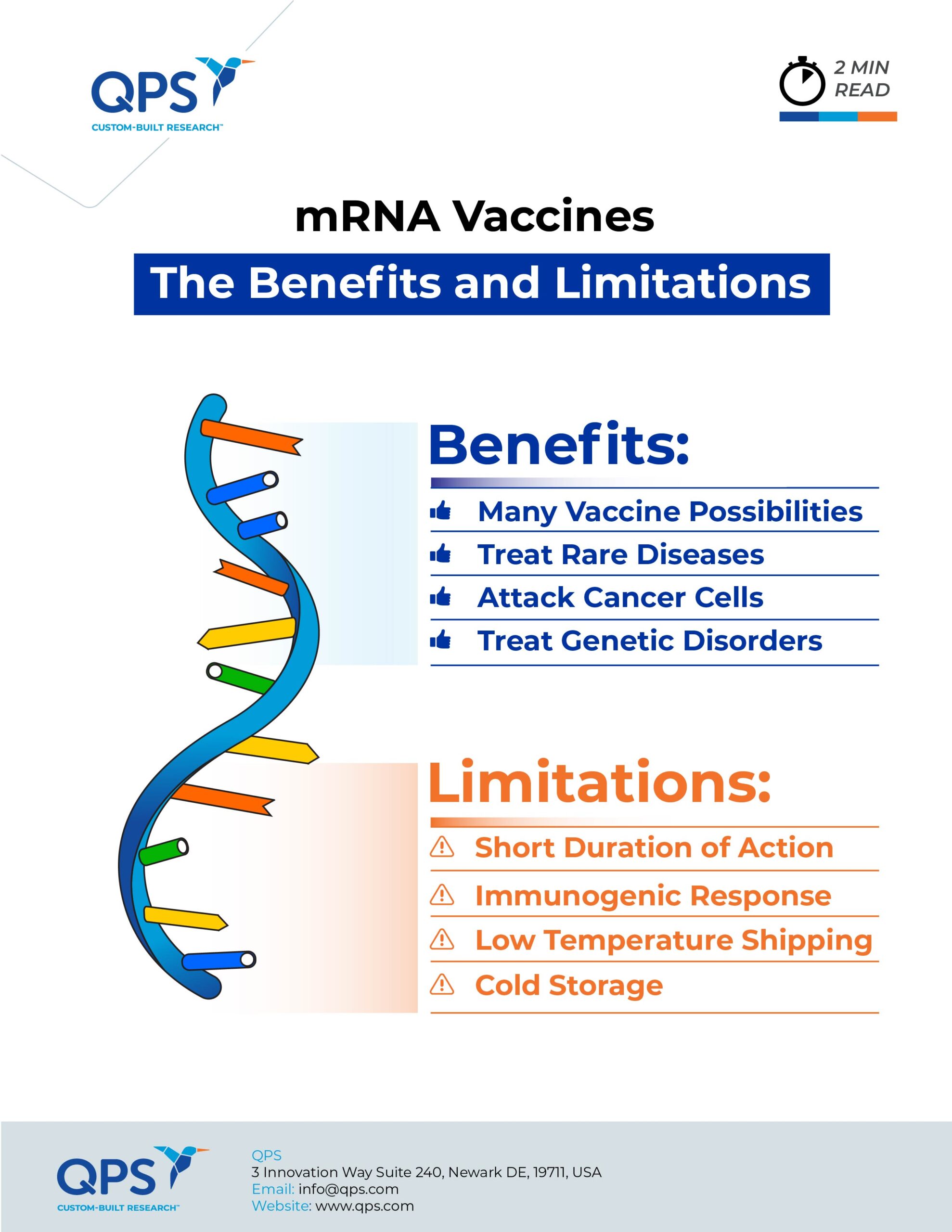
While research on potential therapeutic applications of mRNA has been underway for more than a decade, the success of the Pfizer and Moderna COVID-19 vaccines has expanded interest in the field, with manufacturers like Sanofi announcing significant investment and intent to become an active participant in developing mRNA-based therapies. In addition to developing antibodies as vaccines, researchers have been exploring ways to leverage mRNA’s protein-making abilities to create enzymes to treat rare diseases, as well as agents to promote growth and restore cardiac tissue. While there is a great deal of promise, the breadth and depth of mRNA’s potential application may be limited by some inherent challenges that will need to be addressed. As Derek Lowe observed in a recent commentary in Science News, “mRNA is not a magic road to efficacy.” Dr. Lowe went on to detail some of the challenges of working with mRNA that researchers will need to overcome to realize the full potential of mRNA therapies:
Maintaining a Therapeutic Response
The basic premise behind mRNA vaccines and therapeutics is that mRNA instructs cells to manufacture protein – but as soon as that message is no longer being delivered, manufacturing stops. This isn’t an issue for vaccines in which the manufactured proteins “teach” the immune system to recognize and fight a virus. mRNA-based therapeutics, however, would require ongoing protein production – which would require ongoing mRNA administration. For how long? Clinical trials would need to determine that, while also seeing how ongoing treatment affects safety and efficacy. (More on that later.)
Immunogenic Responses – Friend and Foe
Early research into mRNA therapeutics demonstrated that, like any other foreign object in the human body, manufactured mRNA triggers an immune system response. Unless the mRNA is engineered to fly under the immune system radar, it will be attacked before it can reach the target cells. mRNA vaccines are engineered to decrease an immediate immune reaction so they last long enough to stimulate sustained immunity, while mRNA drugs with an all-human protein sequence might not elicit a full-blown immune response. For some patients, the immune system response may go beyond rendering the mRNA ineffective and basic allergic reactions like rashes. Myocarditis, clotting problems and life-threatening reactions like anaphylactic shock are also possible. Figuring out how to evade immune response will be key to seeing mRNA therapeutics reach their full potential.
Issues with Standard Delivery
Whether administered intravenously, intraperitoneally, intramuscularly, or subcutaneously, synthetic mRNA will break down before it can enter the cell without an engineered, protective carrier. The lipid nanoparticles used to deliver the mRNA in COVID-19 vaccines protect the mRNA but also accumulate in the liver. While not as much of an issue for vaccines, the potential impact on liver health and function is a downside for any mRNA therapy that would require ongoing administration. The upside is mRNA therapies that target liver disease will be very effective in reaching their target. Injecting mRNA drugs directly into the target tissue is one way around this issue – and trials are underway to study injecting mRNA directly into the eyes to treat genetic retinal diseases – but other solutions will be needed to reach cells in less accessible organs.
Great Expectations vs. Limited Applications
Assuming scientists will be able to address the challenges listed above, the final challenge that may limit mRNA’s potential is determining the best use of the technology. The potential for additional mRNA vaccines is evident by the number of manufacturers, like Sanofi, investing to get in the game. Vaccine technology may expand well past infectious disease – one hope is using mRNA to elicit an immune response that would attack cancer cells – so the potential application there should not be underestimated. Beyond the immune response, clinical trials indicate there is a definite sweet spot for mRNA in treating diseases or genetic disorders that are caused by or result in compromised protein creation, structure or function, such as cystic fibrosis, Alzheimer’s disease, and rare diseases including methylmalonic acidemia, acute intermittent porphyria, and Fabry disease.
Time will tell whether this technology will be the answer science has been looking for to prevent and treat a myriad of diseases and conditions … or perhaps genetic engineering technology like CRISPR will do the job more effectively with fewer side effects. As Dr. Lowe summarized so succinctly, exploring the potential of mRNA is “going to be a long story with a lot of plot twists, but I’m glad we’re telling it.”
Did you enjoy this blog post? Check out our other blog posts as well as related topics on our Webinar page.
QPS is a GLP- and GCP-compliant contract research organization (CRO) delivering the highest grade of discovery, preclinical and clinical drug research development services. Since 1995, it has grown from a tiny bioanalysis shop to a full-service CRO with 1,100+ employees in the U.S., Europe and Asia. Today, QPS offers expanded pharmaceutical contract R&D services with special expertise in neuropharmacology, DMPK, toxicology, bioanalysis, translational medicine and clinical development. An award-winning leader focused on bioanalytics and clinical trials, QPS is known for proven quality standards, technical expertise, a flexible approach to research, client satisfaction and turnkey laboratories and facilities. Through continual enhancements in capacities and resources, QPS stands tall in its commitment to delivering superior quality, skilled performance and trusted service to its valued customers. For more information, visit www.qps.com or email info@qps.com.