The events of the COVID-19 pandemic have largely upended how we perceive and respond to global viral threats. One positive change is the technology that has been developed throughout the pandemic’s course and the promise it holds for the future of vaccine development. For example, fears of new pandemics, and experiences with past ones, are now encouraging researchers to develop a broader, more comprehensive preventative response to influenza in an effort to stave off the spread of new and more harmful strains of the virus.
A Novel Way of Confronting an Old Threat
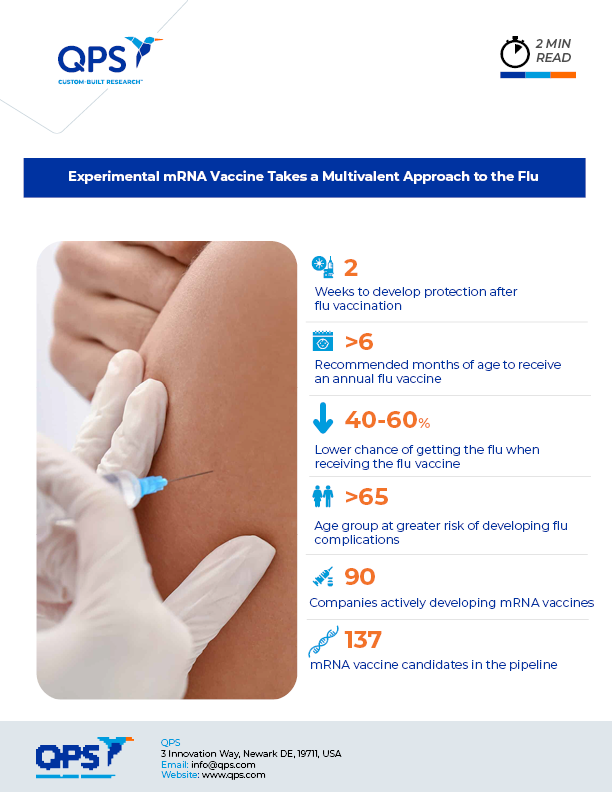
Over the course of the past century, scientists have noted that approximately once every 10 years they see influenza strains “jump” from animals to humans, as was the case with the swine flu (H1N1) pandemic that struck the globe in 2009. These jumps, alongside the constantly mutating influenza virus, stand as the largest threats in future influenza pandemics. Analysts fear that a new, more dangerous strain may emerge from these existing patterns.
A team of scientists are now harnessing mRNA technologies developed to combat COVID-19 with hopes of developing a vaccine that can target more than 20 different variants of influenza. However, the multi-target approach will not alter our annual flu vaccination schedule. The head of the research team, Scott Hensley of the University of Pennsylvania, states that the “kitchen sink” vaccine instead will allow patients to develop immunity to multiple strains of influenza, and even future descendants of present strains.
If the vaccine proves effective, the researchers specifically hope that it will allow younger portions of the population to be afforded time to develop immunity to multiple influenza strains. According to Hensley, it is during childhood exposures to strains of influenza that we develop the greatest immunity to future infections. Therefore, by allowing children to be exposed to the 20 variants, there should be fewer cases of severe infection later in life. By immunizing most children by the age of five, Hensley hopes that we may be able to prevent future infections and subsequent mutations that encourage the development of harmful strains of influenza. Furthermore, the vaccine will allow children to be exposed to strains that are now less prevalent due to historical attempts to stymie their spread.
When Can We Expect the Vaccine?
Though the thought of receiving over 20 different strains of a virus can be disconcerting, Hensley notes that the body regularly processes hundreds of immune system threats, and our response should be no different after receiving a 20-valent injection. The vaccine is currently undergoing tests in ferrets, mice, and nonhuman primates. Trials in humans could begin as soon as early this year, according to the research team.
The next step, according to Hensley, is to figure out just how many antigens (in other words, viral strains) scientists might be able to “fit” into a dose of the vaccine. Future challenges also include the rigorous testing required of a new vaccine, which will be difficult due to the vaccine’s multiple targets. Dr. Dan Barouch, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center in Boston, summarizes future roadblocks: “There may be manufacturing or regulatory challenges for a 20-valent vaccine, but this study shows an important proof-of-concept for multiplexing flu vaccines.”
Only testing and further research will offer an answer regarding a new multivalent mRNA flu vaccine’s efficacy and safety, but it provides promising prospects for future generations and future efforts at preventing another pandemic.
Did you enjoy this blog post? Check out our other blog posts as well as related topics on our Webinar page.
QPS is a GLP- and GCP-compliant contract research organization (CRO) delivering the highest grade of discovery, preclinical and clinical drug research development services. Since 1995, it has grown from a tiny bioanalysis shop to a full-service CRO with 1,200+ employees in the U.S., Europe and Asia. Today, QPS offers expanded pharmaceutical contract R&D services with special expertise in neuropharmacology, DMPK, toxicology, bioanalysis, translational medicine and clinical development. An award-winning leader focused on bioanalytics and clinical trials, QPS is known for proven quality standards, technical expertise, a flexible approach to research, client satisfaction and turnkey laboratories and facilities. Through continual enhancements in capacities and resources, QPS stands tall in its commitment to delivering superior quality, skilled performance and trusted service to its valued customers. For more information, visit www.qps.com or email [email protected].