A groundbreaking gene editing therapy for sickle cell disease from CRISPR Therapeutics and Vertex Pharmaceuticals has received approval from the U.S. Food and Drug Administration. This long-awaited treatment offers hope to people living with the disease and can potentially transform their lives by ending years of debilitating episodes of pain. However, challenges remain, such as the treatment’s high cost, potential side effects, and the need for longer-term data to determine the lasting effects.
 Sickle-Cell’s Unmet Need
Sickle-Cell’s Unmet Need
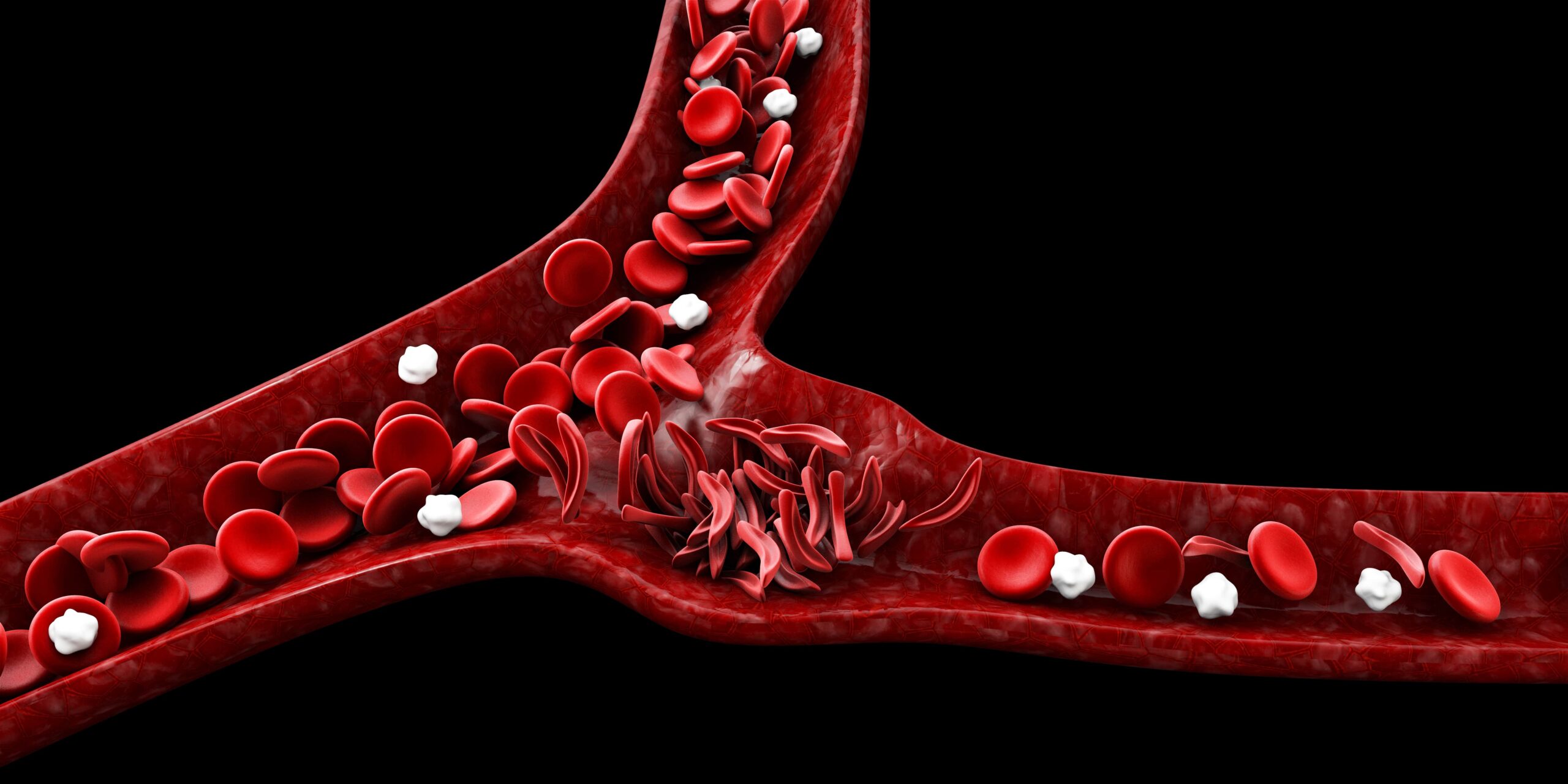
Sickle cell disease is a genetic disorder that primarily affects people of African descent. The condition is characterized by deformed red blood cells that often lead to blockages in the blood vessels, triggering extreme pain episodes known as vaso-occlusive crises. The cause of this disease lies in a mutation in the gene responsible for hemoglobin production. Hemoglobin is the protein tasked with oxygen transportation in red blood cells. The mutation causes the red blood cells to take on a crescent shape and develop a sticky texture, which results in clump formations that hinder blood flow. Sickle cell disease can lead to organ damage and a variety of incapacitating symptoms.
Milestone Treatment
The treatment, known generically as exa-cel, is the first CRISPR-based gene-editing therapy approved in the United States. It is a one-time therapy that combines CRISPR gene editing with stem cell transplantation. To carry out this treatment, stem cells are first harvested from the patient’s bone marrow and transported to a facility where CRISPR technology is used to edit the BCL11A gene precisely.
The edited stem cells are reinfused into the patient’s body, where they develop into red blood cells with fetal hemoglobin. This type of hemoglobin is present in early life and can alleviate the symptoms of sickle cell disease. By increasing the fetal hemoglobin levels in the patient’s red blood cells, exa-cel aims to mitigate the severity of the disease and forestall pain crises.
Although not a complete cure for sickle cell disease, exa-cel has delivered dramatic results. “It was like day and night. I stopped having crises,” said Elinam Joe Tsogbe, who received the treatment in 2021. Now 37 years old, Tsogbe had endured painful crises his entire life and experienced a series of related health complications. He enrolled in the exa-cel clinical trial after researching the therapy and other options for a year. Following the treatment, “There was this newfound energy that was very different. You’re in disbelief, basically,” he said.
Challenges to Overcome
While exa-cel therapy offers immense benefits, it also poses challenges for some patients. The treatment requires a preparatory chemotherapy regimen, which carries potential side effects and may not be suitable for older patients or those with organ damage. In addition, the chemotherapy treatment can lead to infertility. The cost of the treatment may also be a barrier, although a proposed pilot program could make it more affordable for state Medicaid agencies.
Next Steps
Researchers will continue to follow participants in the clinical trial to evaluate the durability and permanence of the treatment’s effects. “A cure would mean all side effects associated with sickle cell disease are either halted or reversed, which we don’t know yet, and that such a change happens to be permanent and last lifelong, which again has not been shown yet,” said Akshay Sharma, a pediatric hematologist at St. Jude’s Children’s Research Hospital in Memphis and a researcher in the exa-cel clinical trial.
Despite these questions, the treatment’s approval and its demonstrated benefits will continue to raise expectations for a significant shift in how genetic conditions are managed in the future.
Did you enjoy this blog post? Check out our other blog posts as well as related topics on our Webinar page.
QPS is a GLP- and GCP-compliant contract research organization (CRO) delivering the highest grade of discovery, preclinical and clinical drug research development services. Since 1995, it has grown from a tiny bioanalysis shop to a full-service CRO with 1,100+ employees in the U.S., Europe and Asia. Today, QPS offers expanded pharmaceutical contract R&D services with special expertise in neuropharmacology, DMPK, toxicology, bioanalysis, translational medicine and clinical development. An award-winning leader focused on bioanalytics and clinical trials, QPS is known for proven quality standards, technical expertise, a flexible approach to research, client satisfaction and turnkey laboratories and facilities. Through continual enhancements in capacities and resources, QPS stands tall in its commitment to delivering superior quality, skilled performance and trusted service to its valued customers. For more information, visit www.qps.com or email info@qps.com.







