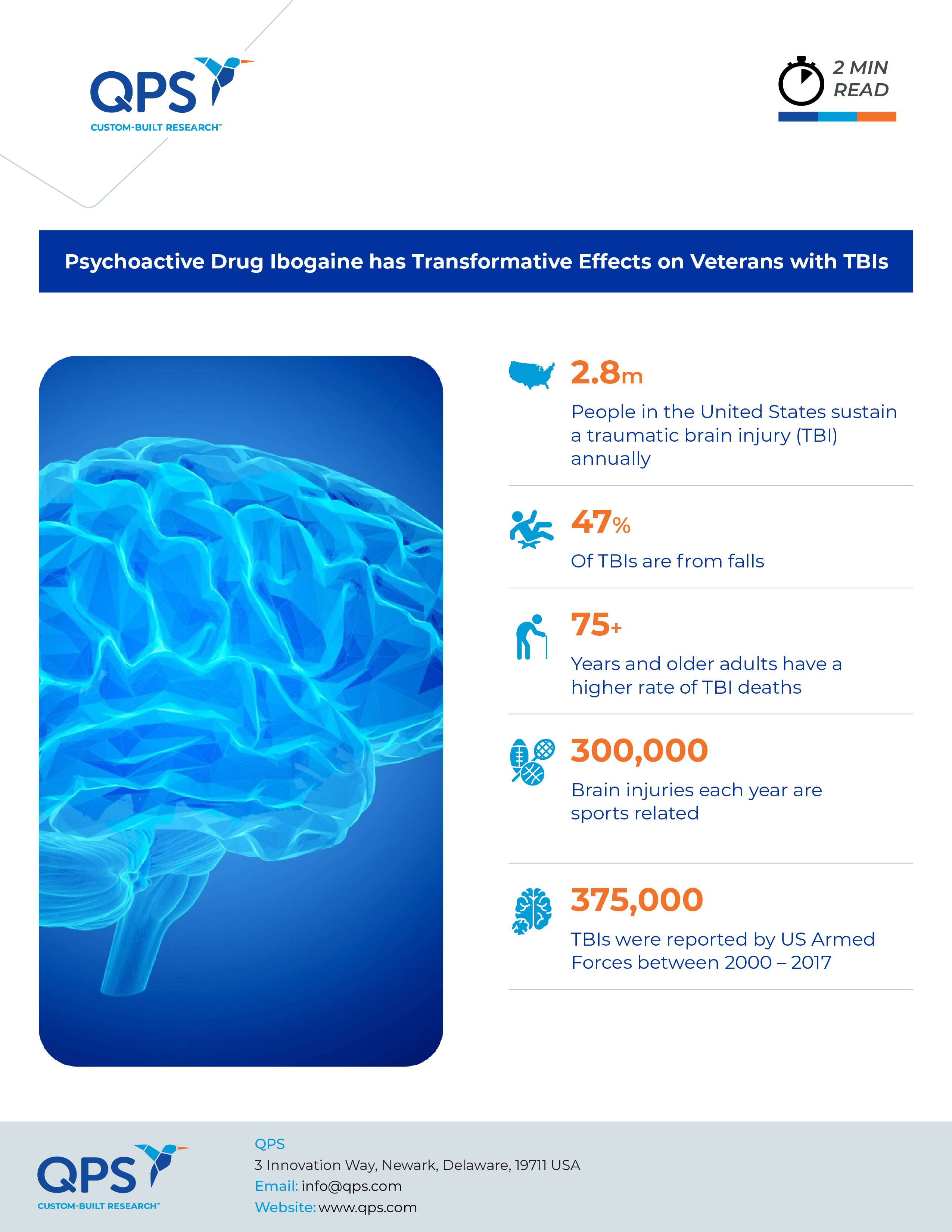
Traumatic brain injuries (TBIs), commonly experienced by military personnel, often incur deep damage and invisible scars. Such injuries are significant contributors to post-traumatic stress disorder (PTSD), anxiety, depression, and suicide rates among veterans. Yet, conventional treatments have frequently fallen short in alleviating the long-term effects of TBIs, leaving many veterans desperate for effective solutions. As psychedelics are gradually gaining mainstream acceptance in mental health treatment, a small study found dramatic results with ibogaine, a plant-based psychoactive compound, among veterans of the Special Operations Forces.
Exploring Alternative Solutions
TBI is defined as a disruption in normal brain function caused by external forces such as explosions or bodily impacts. The resulting trauma can lead to structural and functional changes in the brain, contributing to a myriad of neuropsychiatric symptoms. With hundreds of thousands of U.S. troops sustaining TBIs in recent conflicts, there is an urgent need for effective treatments, particularly for those resistant to mainstream therapies.
Ibogaine is a naturally occurring psychoactive substance found in the roots of the African iboga plant and has been used in religious ceremonies for centuries by indigenous people. Research has demonstrated ibogaine’s efficacy in treating addiction, and the drug is administered in alternative medicine clinics worldwide.
Although ibogaine is a Schedule I substance in the U.S., veterans can access legal ibogaine treatments in Mexico, prompting researchers to investigate its therapeutic potential. “There were a handful of veterans who had gone to this clinic in Mexico and were reporting anecdotally that they had great improvements in all kinds of areas of their lives after taking ibogaine,” said study co-author Nolan Williams, Associate Professor of Psychiatry and Behavioral Sciences at the Stanford School of Medicine. “Our goal was to characterize those improvements with structured clinical and neurobiological assessments.”
A Collaborative Effort
Williams’ team at Stanford collaborated with VETS, a nonprofit organization that helps veterans access psychedelic-assisted therapies for TBI and PTSD, to conduct the study. Thirty male U.S. Special Operations veterans, most of whom were experiencing severe psychiatric symptoms and functional disabilities, received the treatment. Before and after treatment, researchers assessed the participants for symptoms of PTSD, anxiety disorder, and alcohol use disorder and on functional measures such as cognition, self-care, and community participation. The treatment protocol included medical monitoring of ibogaine administration combined with magnesium to mitigate potential heart-related complications.
Promising Results
The research results, published in Nature Medicine, showed that study participants experienced significant improvements in functioning, PTSD, depression, and anxiety symptoms following ibogaine treatment. Importantly, these improvements persisted for at least one month post-treatment, suggesting sustained benefits. Results of cognitive tests showed that the participants experienced improved concentration, memory, and impulsivity. No serious side effects were reported.
One study participant credited the treatment with saving his life. “Before the treatment, I was living life in a blizzard with zero visibility and a cold, hopeless, listless feeling,” said Sean, a 51-year-old veteran from Arizona with six combat deployments. “After ibogaine, the storm lifted.”
“No other drug has ever been able to alleviate the functional and neuropsychiatric symptoms of traumatic brain injury,” said Williams. “The results are dramatic, and we intend to study this compound further.”
Looking Ahead
The researchers will continue to explore the drug’s mechanisms of action and consider a wider population that could benefit from its use. “This may emerge as a broader neuro-rehab drug,” Williams said. “I think it targets a whole host of different brain areas and can help us better understand how to treat other forms of PTSD, anxiety and depression that aren’t necessarily linked to TBI.”
Did you enjoy this blog post? Check out our other blog posts as well as related topics on our Webinar page.
QPS is a GLP- and GCP-compliant contract research organization (CRO) delivering the highest grade of discovery, preclinical and clinical drug research development services. Since 1995, it has grown from a tiny bioanalysis shop to a full-service CRO with 1,100+ employees in the U.S., Europe and Asia. Today, QPS offers expanded pharmaceutical contract R&D services with special expertise in neuropharmacology, DMPK, toxicology, bioanalysis, translational medicine and clinical development. An award-winning leader focused on bioanalytics and clinical trials, QPS is known for proven quality standards, technical expertise, a flexible approach to research, client satisfaction and turnkey laboratories and facilities. Through continual enhancements in capacities and resources, QPS stands tall in its commitment to delivering superior quality, skilled performance and trusted service to its valued customers. For more information, visit www.qps.com or email info@qps.com.