The Antibody-Drug Conjugate Oncology Drug Development Challenge
Antibody-drug conjugates (ADCs) uniquely target cancer cells by combining the precision of monoclonal antibodies, which identify the cancer cells, with the potent cell-killing ability of cytotoxic agents. Both entities, namely the antibody and the cytotoxic agent, are already therapies for a certain type of cancer, but combining them enhances the efficacy and safety profiles. Breakthroughs in antibody engineering, novel linker chemistries and advanced payload design (more molecules per antibody) have catalyzed a surge in ADC development. This new generation of ADCs is achieving improved efficacy and more manageable side effects, making them a promising frontier for cancer treatment.
For example, Phase III clinical trial results show that trastuzumab deruxtecan, an ADC with eight molecules of deruxtecan linked to one trastuzumab molecule, approved for human epidermal growth factor receptor 2 (HER2)-positive breast cancer, results in significantly longer progression-free survival and overall survival in patients with HER2-low metastatic breast cancer compared to standard chemotherapy.
This white paper will explore the evolving landscape of ADCs, highlighting key technological advancements, regulatory considerations and clinical trends. It will also discuss challenges and opportunities in ADC development and how QPS’ expertise can support the successful advancement of ADC programs.
WHAT IS AN ANTIBODY-DRUG CONJUGATE?
- Antibodies: These provide high specificity for tumor-associated antigens, ensuring that the therapeutic effect is focused on cancer cells while minimizing off-target effects.
- Linkers: These specialized chemical structures attach the cytotoxic payload to the antibody. Linkers are engineered to remain stable in the bloodstream, preventing premature release, yet are designed to break and release the payload once the ADC is internalized into the target cancer cell.
- Cytotoxic Payloads: Each antibody carries multiple molecules of these highly potent agents, capable of inducing cell death with minimal quantities per kg body weight. This strategy ensures that even a small number of targeted cancer cells are effectively destroyed.
EARLY ADCs: THE FOUNDATION FOR A RAPIDLY ADVANCING FIELD
The concept of arming antibodies with cytotoxic payloads to selectively target cancer cells dates back to the 1980s. The first ADC to gain FDA approval, gemtuzumab ozogamicin for acute myeloid leukemia, reached the market in 2000. However, it was voluntarily withdrawn in 2010 due to safety concerns before being reapproved in 2017 with an optimized dosing regimen. In 2011, the approval of brentuximab vedotin for Hodgkin’s lymphoma marked an important milestone, ushering in a new wave of ADC development.
Since then, the pace of development has accelerated. Advancements in antibody engineering, linker chemistry and payload selection have dramatically improved ADC efficacy and safety. Worldwide, 17 ADCs have been approved (see Table), and hundreds more are in clinical development for various oncology indications.
KEY TECHNOLOGICAL ADVANCES IN ADC DEVELOPMENT
Antibody Engineering
Linker Chemistry
Payload Design and Conjugation
Developments in payload design and site-specific conjugation allow ADCs to deliver their cytotoxic load more efficiently. These advances boost the therapeutic index by reducing systemic toxicity while increasing on-target potency.
REGULATORY CONSIDERATIONS FOR ADCs
In March 2024, the US Food and Drug Administration (FDA) issued a Guidance for Industry on the Clinical Pharmacology Considerations for Antibody-Drug Conjugates. The FDA Guidance provides recommendations related to the development of ADCs with a cytotoxic small-molecule drug or payload. It includes considerations in the following areas: bioanalytical methods, dosing strategies, dose-and exposure-response analysis, intrinsic factors, QTc assessments, immunogenicity and drug-drug interactions.
In December 2024, the National Medical Products Administration’s Center for Drug Evaluation published a Technical Guideline for Antibody-Drug Conjugate Pharmaceutical Study and Evaluation in China. The European Medicines Agency has not published ADC-specific guidelines but does have Guidelines Relevant for Advanced Therapy Medicinal Products.
CURRENT PHARMACEUTICAL COMPANY ACTIVITY IN ADC DEVELOPMENT
The clinical trial landscape for ADCs is rapidly evolving. Hundreds of ADCs are currently in clinical development, evaluating therapies against a range of tumor types, as shown in Figures 1 and 2. The current inventory of ADCs in development reflects the market landscape, with non-small-cell lung, breast, ovarian, gastric and esophageal cancers at the forefront. This trend is not surprising, considering the proven effectiveness of ADCs in the market.
ADC Trials by Phase and Tumor Type

ADC Trials by Phase and Tumor Type
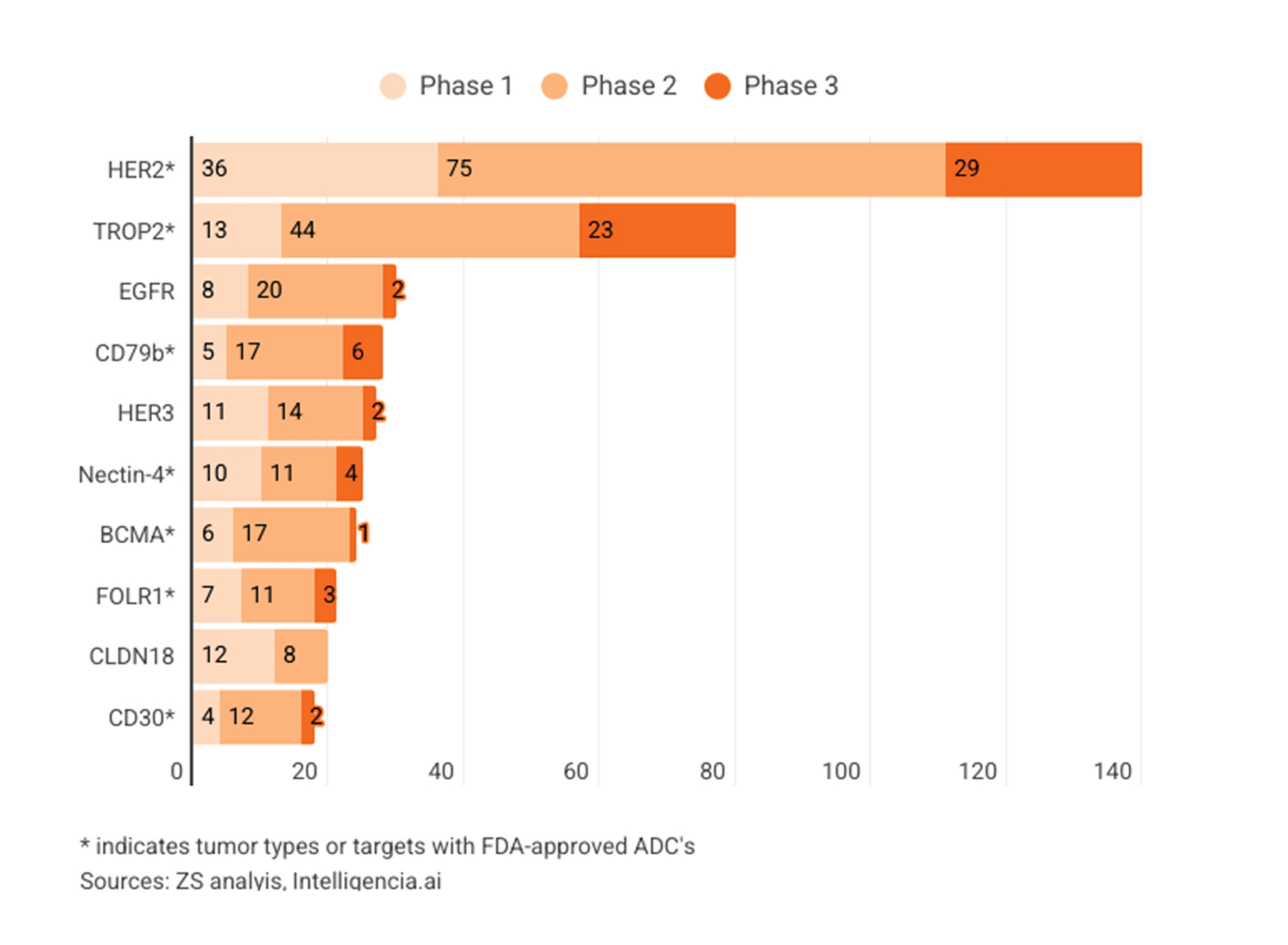
Figures 1 and 2: ADC tumor types and targets by phase of development
Safety and Off-Target Toxicity
Drug Resistance
Regulatory Complexity
The regulatory environment for ADCs continues to evolve. Due to their hybrid biological-chemical composition and high toxicity payload, each component introduces specific development and regulatory considerations. For example, the stability of the linker and controlled payload release are essential in ADC development. Premature release leads to toxicity, while inadequate release reduces efficacy.
ADCs must navigate both biologics and small molecule regulatory guidelines due to their dual nature, complicating the approval process. ADC clinical trial designs need to demonstrate targeted delivery, controlled release and the minimization of systemic toxicity. Adaptive trial designs incorporating biomarker-driven patient selection and efficacy endpoints are increasingly favored by regulatory agencies to expedite approvals.
QPS: YOUR PROVEN PARTNER FOR ADC DEVELOPMENT
Our Capabilities Include:
- Global Clinical Team: Our team includes board-certified oncologists, experienced clinical research coordinators, project managers and skilled data analysts, ensuring deep expertise in ADC development.
- Patient-Centric Approach: We leverage strong collaborations with study sites across Europe, the US and Asia, employing innovative patient recruitment strategies and partnerships.
- State-of-the-Art Facilities: Our advanced bioanalysis laboratories utilize cutting-edge technology for precise method development and assay execution. We have extensive experience conducting pharmacokinetic and immunogenicity ADC projects using ELISA, ECL and (Hybrid) LC-MS/MS techniques.
- Customized Oncology Solutions: We design tailored study protocols to meet the unique needs of oncology research, including biomarker analysis, imaging services and specialized assay development.
- Proven Track Record: We have successfully conducted global Phase I-IV oncology studies, covering a broad range of cancers such as breast, head and neck, lung and prostate.
ADVANCING ADCs INTO THE FUTURE
The rapid evolution of ADC technology is setting a new standard in targeted cancer therapy. With significant advancements in antibody design, linker stability and payload potency, the next generation of ADCs is poised to deliver greater clinical efficacy and safety. As the ADC pipeline continues to grow and diversify, QPS remains a trusted partner, offering the specialized expertise and integrated solutions needed to navigate this complex landscape. Together, we can accelerate the development of transformative therapies that offer renewed hope for patients worldwide.
REFERENCES
K. Liu, M. Li, Y. Li, et al. A review of the clinical efficacy of FDA-approved antibody-drug conjugates in human cancers. Molecular Cancer, vol. 23, 2024, p. 62. doi:10.1186/s12943- 024-01963-7. https://doi.org/10.1186/s12943-024-01963-7. Accessed 3 March 2025.
M. Furlow, C. Corridon, B. Coyle, and R. Bonnot. Oncology’s next revolution: Antibody-drug conjugates and how to push them into the future, 2024. ZS Associates. https://www.zs.com/insights/oncology-antibody-drug-conjugates-revolution. Accessed 3 March 2025.
Riccardi, Federico, et al. A comprehensive overview on antibody-drug conjugates: from the conceptualization to cancer therapy. Frontiers in Pharmacology, vol. 14, 18 Sept. 2023, p. 1274088. doi:10.3389/fphar.2023.1274088. https://pmc.ncbi.nlm.nih.gov/ articles/PMC10544916/. Accessed 3 March 2025.
Sasso, J. M., Tenchov, R., Bird, R., Iyer, K. A., Ralhan, K., Rodriguez, Y., and Zhou, Q. A. The Evolving Landscape of Antibody-Drug Conjugates: In Depth Analysis of Recent Research Progress. Bioconjugate Chemistry, vol. 34, no. 11, 2023, pp. 1951–2000. doi:10.1021/acs.bioconjchem.3c00374. https://doi.org/10.1021/acs.bioconjchem.3c00374. Accessed 3 March 2025.
https://www.zs.com/insights/oncology-antibody-drug-conjugates-revolution





