Introduction
In short, RNA therapies modulate gene expression and regulate targeted disease-causing proteins. They offer several advantages over other therapies due to their specificity and versatility, and have enabled researchers to make great strides toward personalized medicine.
MESSENGER RNAs (mRNAs)
Messenger RNA is a type of RNA molecule that serves as the intermediate carrier of genetic information from the DNA to the ribosomes, where it is translated into protein structures. The mRNA molecule is transcribed from the DNA sequence through a process called genetic transcription and carries this genetic code from the nucleus to the cytoplasm of the cell.
Synthetic mRNA is a single-stranded nucleotide sequence consisting of five regions (Figure 1). The functional section is the open reading frame (ORF) where the transcription for protein biosynthesis is accomplished. On either, end of the ORF, are the 5′ and 3′ untranslated regions (UTRs). These regions control translation efficacy and decay rate of the mRNA. The 5′ endcap protects the mRNA from ribonucleases and the polyadenine end group added to the 3′ end regulates mRNA translation efficacy and protein expression.1, 2
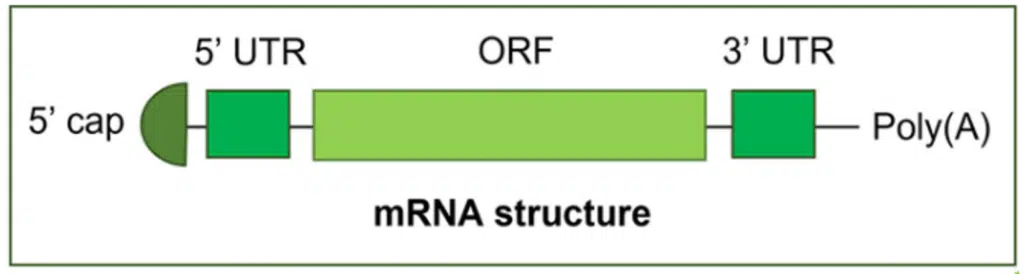
Figure 1. Structure of a typical mRNA drug. (Image courtesy of Ref. 2)
The optimization of both mRNA structure and the transport system are essential for success of the mRNA therapeutic. During translation, mRNA interacts with the ribosomes and acts as a template for the synthesis of proteins. Ribosomes read the mRNA codons and match them with the corresponding transfer RNA (tRNA) molecules that generate a specific amino acid sequence. This process leads to the assembly of a polypeptide chain, which folds into a functional or in this case dysfunctional protein.2
SMALL INTERFERING RNAs (siRNAs) AND ANTISENSE OLIGONUCLEOTIDES (ASOs)
Small Interfering RNAs are double-stranded RNA molecules of about 20 to 25 base pairs in length that can target and degrade specific sequences of mRNA molecules, thereby silencing the expression of disease-causing genes. This class of RNA is “hybridized” with a sense or “guide” strand, which facilitates transport through cell membranes to the site of action. The siRNA design targets specific mRNA sequences corresponding to a particular gene of interest, ultimately affecting the outcome of the disease.3
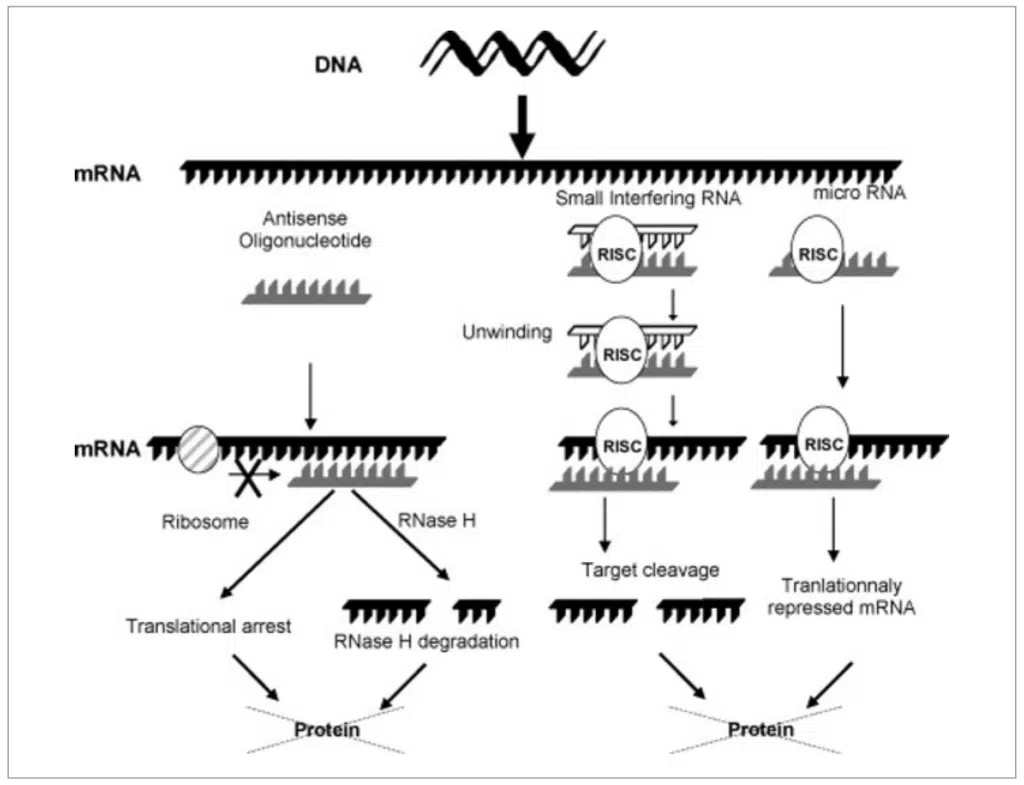
Figure 2. Mechanisms of ASO, siRNA and microRNA disruption of targeted protein synthesis (Image courtesy of Ref. 3)
Antisense oligonucleotides (ASOs) are a class of single-stranded synthetic RNA or DNA molecules designed to specifically bind to and affect the expression of target genes. ASOs are designed to hybridize with complementary mRNA molecules and inhibit their translation or promote their degradation. ASOs can target a wide range of RNA molecules, including both coding and non-coding RNAs. They can target pre-mRNA, mature mRNA, or other RNA species involved in gene regulation. 3, 4
RNA APTAMERS AND MicroRNAs
RNA aptamers are single-stranded RNA molecules consisting of 30 to 90 base pairs with well-defined three- dimensional structures that specifically bind to and inhibit proteins or other small molecules. The secondary and tertiary structure of aptamers, including loops, bulges, and hairpins, target molecules with high affinity and specificity. MicroRNAs are single-stranded RNAs similar to aptamers in that they folded back on themselves but are for the most part linear and do no not possess the fine secondary and tertiary structure as do the aptamers. They are also known as chemical antibodies due to their similarity in action modes to antibody mechanisms. Aptamer-based therapeutics include antagonist aptamers that disrupt the interaction between disease-associated targets and cell-specific aptamers that can serve as carriers to transport other therapeutic agents to the target cells. 3, 5
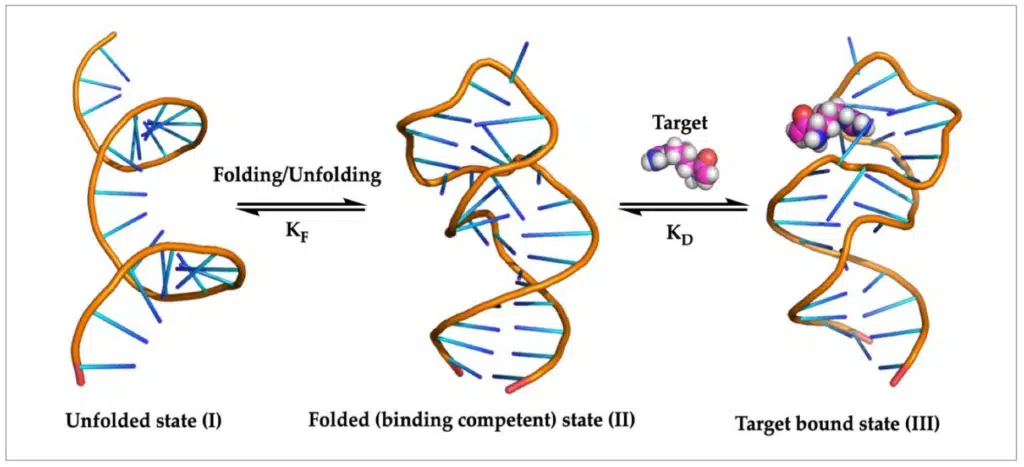
Figure 3. RNA aptamer in unfolded, folded and target bound equilibrium states. (Image courtesy of Ref. 5)
THE FUTURE OF THERAPEUTIC RNAs
The success of mRNA-based vaccines against COVID-19 has already shown them to be effective therapeutics. Researchers now are exploring the use of RNA vaccines for a wide range of infectious diseases including influenza, HIV, and emerging infectious diseases. These new vaccines provide instructions to cells to produce viral antigens triggering an immune response. In the future, mRNA therapies will extend beyond infectious diseases to treat cancer, autoimmune disorders, and other conditions.1, 2, 4 RNA interference will continue to be a powerful mechanism to selectively silence or downregulate the expression of specific genes. Paired with the revolutionary gene-editing CRISPR-Cas9 technology, the utility of RNA molecules designed to modify the genome will be greatly expanded. CRISPR-associated RNA will enable targeted gene editing and ultimately facilitate treatments of genetic diseases.3
One of the key challenges for therapeutic RNA development is efficient delivery to specific tissues. Researchers are actively investigating various delivery methods, including lipid nanoparticles, viral vectors, and exosomes to improve RNA stability, uptake, and target specificity. RNA drug safety considerations, quality control, and regulatory issues will continue to play a vital role in drug development. Regulatory agencies actively monitor all new developments in the field so they can establish appropriate guidelines.
The future of therapeutic RNA holds great promise in revolutionizing the field of medicine, from gene silencing and mRNA-based vaccines to gene editing and ultimately personalized medicine. Continued research, technological advancements, and regulatory support will be instrumental in realizing the full potential of therapeutic RNAs and improving patient outcomes.
BROAD ACCESS
QPS provides clients with broad access to our nonclinical and clinical development capabilities. Clients also benefit from our experience in nonclinical and clinical development of a diverse portfolio of treatment modalities for a wide range of therapeutic indications, including gene therapy and oncology. Our preferred vendor agreements also provide for the establishment of client-dedicated units within our organization.
TIMELY DELIVERY
Partnering with QPS will position your company for success, enabling timely, personalized delivery of your drug portfolio to the marketplace.
REFERENCES
1. Qin S, Tang X, Chen Y, Chen K, Fan N, Xiao W, Zheng Q, Li G, Teng Y, Wu M, and Song X, “mRNA-based therapeutics: powerful and versatile tools to combat diseases”, Signal Transduct Target Ther. (2022); 7: 166
2. Duan L-J, Wang Q, Zhang C, Yang D-X and Zhang X-Y “Potentialities and Challenges of mRNA Vaccine in Cancer Immunotherapy.” Front. Immunol. 47 (May 2022) 92366.
3. E. Fattal, A. Bochat “State of the art and perspectives for the delivery of antisense oligonucleotides and siRNA by polymeric nanocarriers”, Internat. J. of Pharmaceutics 364, (2008), 237-248
4. Khorkova, O., Stahl, J., Joji, A. et al. “Amplifying gene expression with RNA-targeted therapeutics”. Nat Rev Drug Discov. (2023), 1-23. https://doi. org/10.1038/s41573-023-00704-7.
5. Elskens, J.P.; Elskens, J.M.; Madder, A. “Chemical Modification of Aptamers for Increased Binding Affinity in Diagnostic Applications: Current Status and Future Prospects”. Int. J. Mol. Sci. (2020), 21, 4522. https://doi.org/10.3390/ijms21124522






