Clinical Research Services
At QPS, a global full-service Contract Research Organization (CRO), clinical research services encompass comprehensive support for the development and approval of new therapies across all phases of clinical development. These services typically include study design, site selection, regulatory affairs, patient recruitment, clinical trial management, data collection and analysis, pharmacovigilance, and biostatistics, supported by robust laboratory services, such as preclinical studies (pharmacology and DMPK) and bioanalysis. With offices in USA, Europe, Asia, Australia, and India, and a site network of over 700 locations, QPS leverages local expertise and regulatory knowledge to streamline trial execution across multiple regions, ensuring compliance with international standards. By integrating cutting-edge technology and therapeutic expertise, QPS helps pharmaceutical, biotechnology, and medical device companies accelerate timelines, maintain excellence and bring safe, effective treatments to market more efficiently.
All services are conducted in accordance with ICH-GCP standards (International Conference on Harmonization-Good Clinical Practice) and local regulatory requirements to ensure consistent data integrity and patient safety.
Comprehensive Clinical Trial Management
QPS supports every stage of clinical development with full Phase I–IV trial services. Our end-to-end support includes:
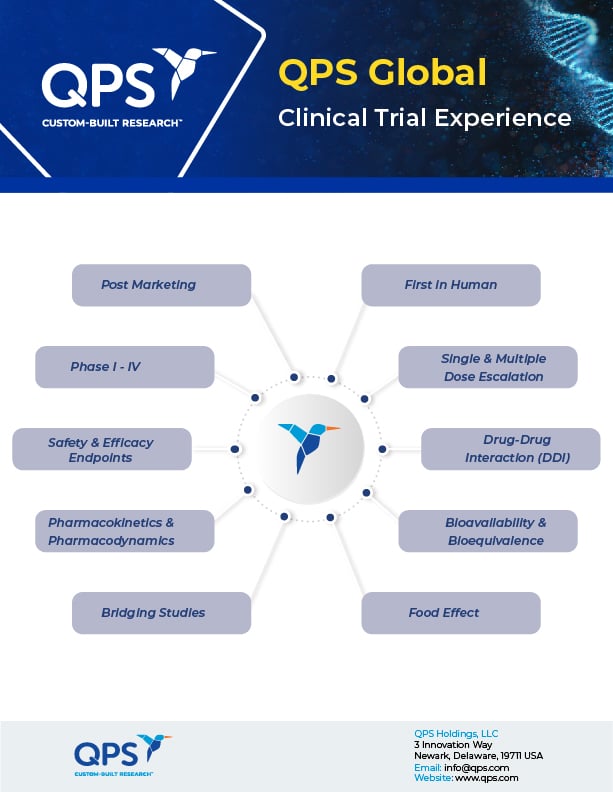
- Phase I - IV Trials
- First-in-Human Trials
- Pharmacokinetics & Pharmacodynamics
- Bioavailability & Bioequivalence
- Drug-Drug Interaction (DDI) Studies
- Food Effect Studies
- Single & Multiple Dose Escalation (SAD/MAD)
- Efficacy Endpoints
- Bridging Studies
- Basket Studies
- Post-Marketing Studies
- Real World Data (RWD) & Real World Evidence (RWE)
Whether launching an early-phase trial or evaluating post-market safety, we tailor services to each protocol for reliable, reproducible outcomes.
Clinical Trial Types and Capabilities
QPS provides full-service clinical trial support across a wide range of study designs and scientific needs. Our capabilities include:
- Phase I - IV Trials: Comprehensive trial management for every clinical development stage.
- First-in-Human Trials: Safely transitioning innovative therapies from preclinical to clinical stages.
- Pharmacokinetics & Pharmacodynamics: Studying how drugs interact with the body and their mechanisms of action.
- Bioavailability & Bioequivalence: Measuring absorption and efficacy of pharmaceutical formulations.
- Drug-Drug Interaction (DDI) Studies: Assessing safety and efficacy when drugs are combined.
- Food Effect Studies: Evaluating how food impacts drug pharmacokinetics.
- Single & Multiple Dose Escalation (SAD/MAD): Determining optimal dosage levels through rigorous study protocols.
- Efficacy Endpoints: Precise evaluation to ensure trial outcomes meet regulatory and scientific benchmarks.
- Bridging Studies: Ensuring clinical data applicability across diverse populations.
- Basket Studies: Early stage oncology clinical trials conducted to determine how a drug works in patients who have different types of cancer that all have the same mutation or biomarker.
- Post-Marketing Studies: Supporting the safety evaluation of drugs after they reach the market.
- Real World Data (RWD) & Real World Evidence (RWE): RWD studies gather the raw data collected from real-world sources (patients or databases), while RWE studies deliver the evidence generated from analyzing and interpreting that data.
QPS Phase I-IV Clinical Services
Partner with QPS Today
Let QPS accelerate your custom-built global drug development program with our comprehensive, full service clinical research services offerings. Contact Us today to get started.
Core Clinical Research Services
Project Management
Centralized coordination to meet timelines and budgets through dedicated project managers.
Site Identification & Qualification
Global site selection based on therapeutic alignment, feasibility, and enrollment potential.
Study/Medical Monitoring
On-site and remote monitoring to ensure protocol adherence, patient safety, and data accuracy.
Pharmacovigilance
Real-time signal detection, SAE tracking, and regulatory reporting to ensure subject safety.
Medical Affairs
Ongoing clinical oversight, medical review, and medical consulting throughout the study.
Protocol Design & Writing
Compliant, scientifically sound protocol development aligned with regulatory agency expectations.
Medical Writing
Regulatory-compliant, scientifically accurate documentation across all study phases.
Biostatistics
Robust trial design, statistical analysis, and interpretation services to support regulatory filings.
Data Management
Validated, audit-ready data capture and management with built-in quality checks.
Quality Assurance
Upholding the highest standards to ensure data integrity and compliance.
Specialized Clinical Trial Support Services
Clinical Trial Kits
Custom trial kits with global distribution and quick turnaround to support complex logistics.
Leukopak Services
Blood donation center with comprehensive Leukopak products and services.
PBMC Laboratories
A one-stop-shop for vaccine studies with on-site PBMC laboratories and trained technicians.
Central Safety Lab
Lightning fast safety lab results with on-site technicians and equipment.
Sample Analysis
High throughput capacity with over 2,000,000 samples analyzed every year.
Bioanalytical Method Development

Why Choose QPS for Clinical Research?
- 30 years of excellence in delivering global, full service CRO operations to pharma and biotech clients.
- Strong relationships with global KOL networks and more than 700 global study sites.
- Service delivery in North and South America, Europe, Asia, Australia, and India.
- Expertise in both drug development research and medical device trials.
- Flexible support from single-country to global studies.
- Integrated data, regulatory, and medical teams.
Links to Relevant Documents
- Efficiently Performing Two Global Approval Studies in Prostate Cancer Patients
- Phase II-IV Clinical Overview
- Phase II-IV Clinical Services & Locations
- Early Phase Clinical Overview
- Expedited Study Delivery – Logistics
- Expedited Study Delivery – Pharmacy
- Studying Sedatives in Phase I Studies
- Pharmacokinetic Studies in Patients
- Corporate Overview Clinical Sites
Let’s Discuss Your Clinical Research Needs
QPS is ready to support your clinical trial with full-service solutions tailored to your timeline, therapeutic area, and regulatory requirements.