Since SARS-CoV-2 (or the novel coronavirus, the virus that causes COVID-19) emerged, many have compared it to the seasonal flu. The seasonal flu virus and SARS-CoV-2 can present with similar symptoms: both seasonal influenza strains (flu A and flu B) and SARS-CoV-2 are contagious viruses that cause respiratory illnesses in humans.
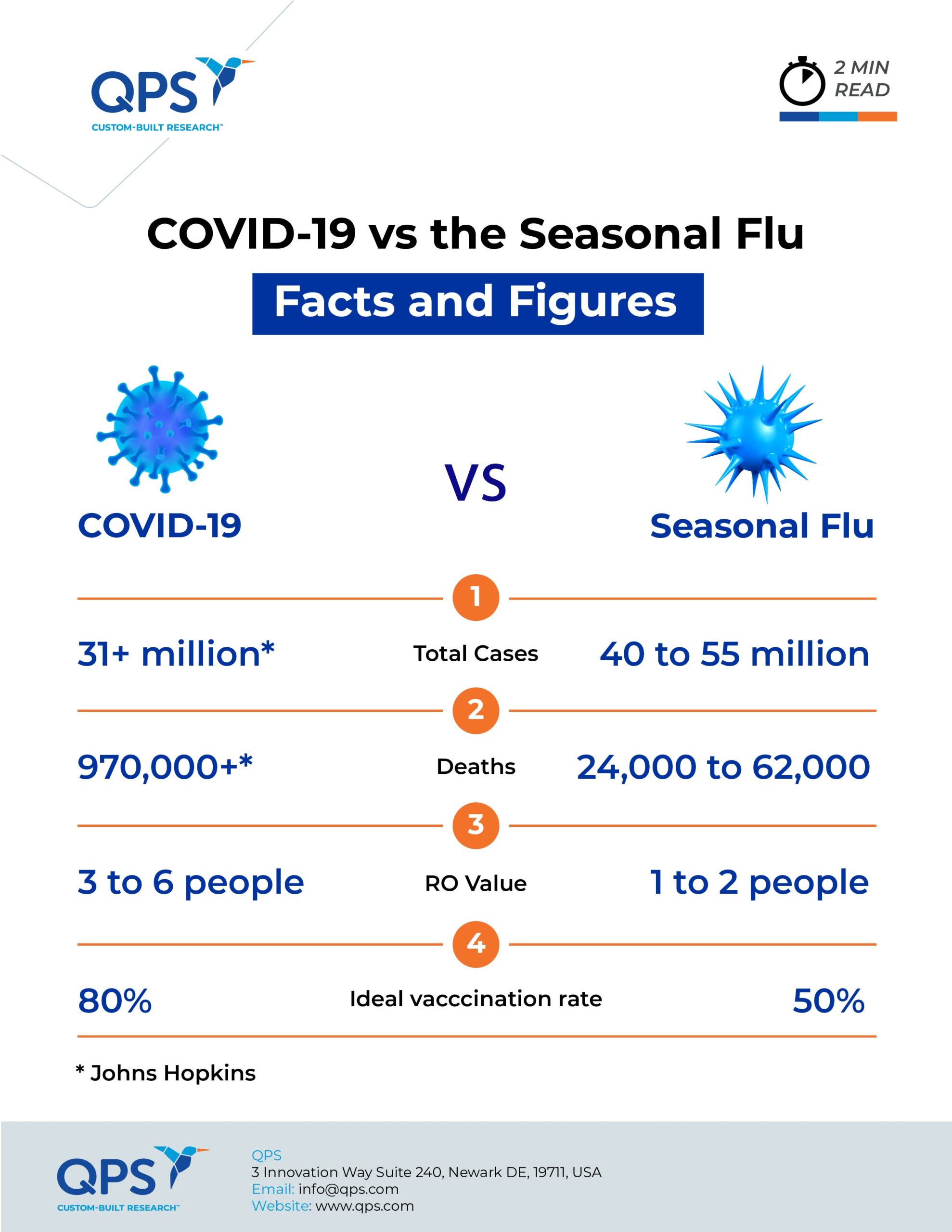
The outcomes of the illnesses, however, are disparate. During last year’s flu season, the CDC estimates there were 40–55 million flu cases and 24,000–62,000 deaths. Johns Hopkins estimates there have been more than 31 million coronavirus cases globally, resulting in more than 970,000 deaths — including over 200,000 deaths in the U.S.
With cold and flu season now here, and the pandemic still growing in many parts of the world, every sneeze and sore throat can be cause for concern. Since the two viruses present with very similar symptoms and physicians do not routinely test for the flu, they can be difficult to distinguish. As physicians and researchers learn more about the novel coronavirus, they have identified many similarities and differences between the two viruses and the illnesses they cause.
Symptoms and Severity
Seasonal flu symptoms include fever, cough, sore throat, muscle aches, headaches, a runny nose and fatigue. These symptoms often appear suddenly, and most seasonal flu patients will recover in two weeks. But for at-risk people — including children, immunocompromised patients and those over the age of 65 — there can be complications, including pneumonia.
Doctors and researchers are still trying to obtain a complete picture of COVID-19 symptoms, which have been reported as ranging from mild to severe. Symptoms most commonly include fever, cough and shortness of breath. Less frequently reported symptoms include chills, shaking, muscle pain, headache and loss of taste or smell. In addition, COVID-19 symptoms develop more gradually than flu symptoms, and worryingly, it seems that there is a large cohort of people who are infected yet exhibit no symptoms at all (asymptomatic infections).
Children are at high risk of complications from the flu, and while their response to infection with the novel coronavirus is still being assessed, the risk of complications must be carefully considered. Although most children do not appear to experience severe COVID-19 symptoms, multisystem inflammatory syndrome in children (MIS-C) has been reported and is a serious concern. This syndrome can affect the heart, lungs, kidneys, brain, skin, eyes and gastrointestinal tract.
Virus Transmission
The “basic reproduction number,” R0 (pronounced r-nought), is the measure used to determine how easily a virus spreads. This is the estimate of the average number of people who catch the virus from a single infected person. The flu has an R0 value of 1.3, which means each person with the flu transmits it to between one and two people. Researchers are still determining the R0 for COVID-19, but preliminary studies according to a JAMA article say an infected person spreads the virus to an average two or three people, giving SARS-CoV-2 an R0 of 2 to 3. Some studies, however, estimate the R0 value to be closer to 6 — one infected person passes the virus on to six people, an infection rate much higher than that of the seasonal flu.
If the R0 of a virus is 2, 50 percent of the population needs to be immunized to stop its spread. But an R0 of 5 means that 80 percent of the population must be immunized; this will likely be the case for SARS-CoV-2 based on the estimated R0.
Hospitalization and Death Rate
Certain portions of the population (children and those over age 65) are more susceptible to the flu, whereas it appears that anyone can contract SARS-CoV-2. In particular, COVID-19 has a large impact on the elderly and non-white populations, which much higher infection rates and more deaths. Whereas the flu only affects 8 percent of the population each year, COVID-19 is on track to infect 50 to 80 percent of the population. The hospitalization rate during the 2019 flu season was 69 hospitalizations per 100,000 people. The overall cumulative COVID-19 hospitalization rate is more than double, at 170.4 per 100,000.
The CDC calculates mortality rates based on death certificates, thus these figures for both the flu and SARS-CoV-2 are only estimates. The overall death rate for the seasonal flu in 2019 was around 0.1 percent in the U.S. There is no singular death rate for COVID-19, but the CDC reports that the percentage of deaths in the U.S. attributed pneumonia, influenza, or COVID-19 in the week ending September 12, 2020 was 6.2 percent. This percentage remains above the epidemic threshold and will likely increase as more death certificates are processed. Fatality rate varies by location, age of person infected and the presence of comorbidities. Additionally, not everyone who has COVID-19 is officially diagnosed. This is partially due to testing limitations — both availability and the fact that those with mild to moderate symptoms may be ineligible for testing. Researchers from Columbia University estimated that only 1 in 12 cases of COVID-19 in the U.S. are documented.
Flu and COVID-19 Treatment and Prevention
Antiviral drugs such as amantadine and rimantadine, as well as influenza inhibitors such as oseltamivir and zanamivir, have proven effective against the flu. Physicians and researchers are trying a variety of treatments against COVID-19, but no known antiviral drugs have proven effective.
Researchers are still at least months away from identifying the safety and efficacy of a viable vaccine for SARS-CoV-2 through clinical trials. In the meantime, the public can protect themselves by wearing a face covering, observing social distancing guidelines and washing their hands frequently, and getting the flu vaccine.
The flu vaccine is widely available and is the best protection for the public against the flu. Getting the vaccine — ideally before the end of October — will also help reduce the strain on healthcare systems as they respond to the COVID-19 pandemic.
QPS is a GLP- and GCP-compliant contract research organization (CRO) delivering the highest grade of discovery, preclinical and clinical drug research development services. Since 1995, it has grown from a tiny bioanalysis shop to a full-service CRO with 1,100+ employees in the U.S., Europe and Asia. Today, QPS offers expanded pharmaceutical contract R&D services with special expertise in neuropharmacology, DMPK, toxicology, bioanalysis, translational medicine and clinical development. An award-winning leader focused on bioanalytics and clinical trials, QPS is known for proven quality standards, technical expertise, a flexible approach to research, client satisfaction and turnkey laboratories and facilities. Through continual enhancements in capacities and resources, QPS stands tall in its commitment to delivering superior quality, skilled performance and trusted service to its valued customers. For more information, visit www.qps.com or email info@qps.com.