Within every hair follicle are the stem cells programmed to grow hair. These cells inhabit and grow in a region of the follicle called the bulge and follow a cycle: hair is generated from the stem cells, the follicle becomes inactive, the hair stops growing, falls out, and then the cells start growing a new strand of hair.
Restarting a Stopped Cycle
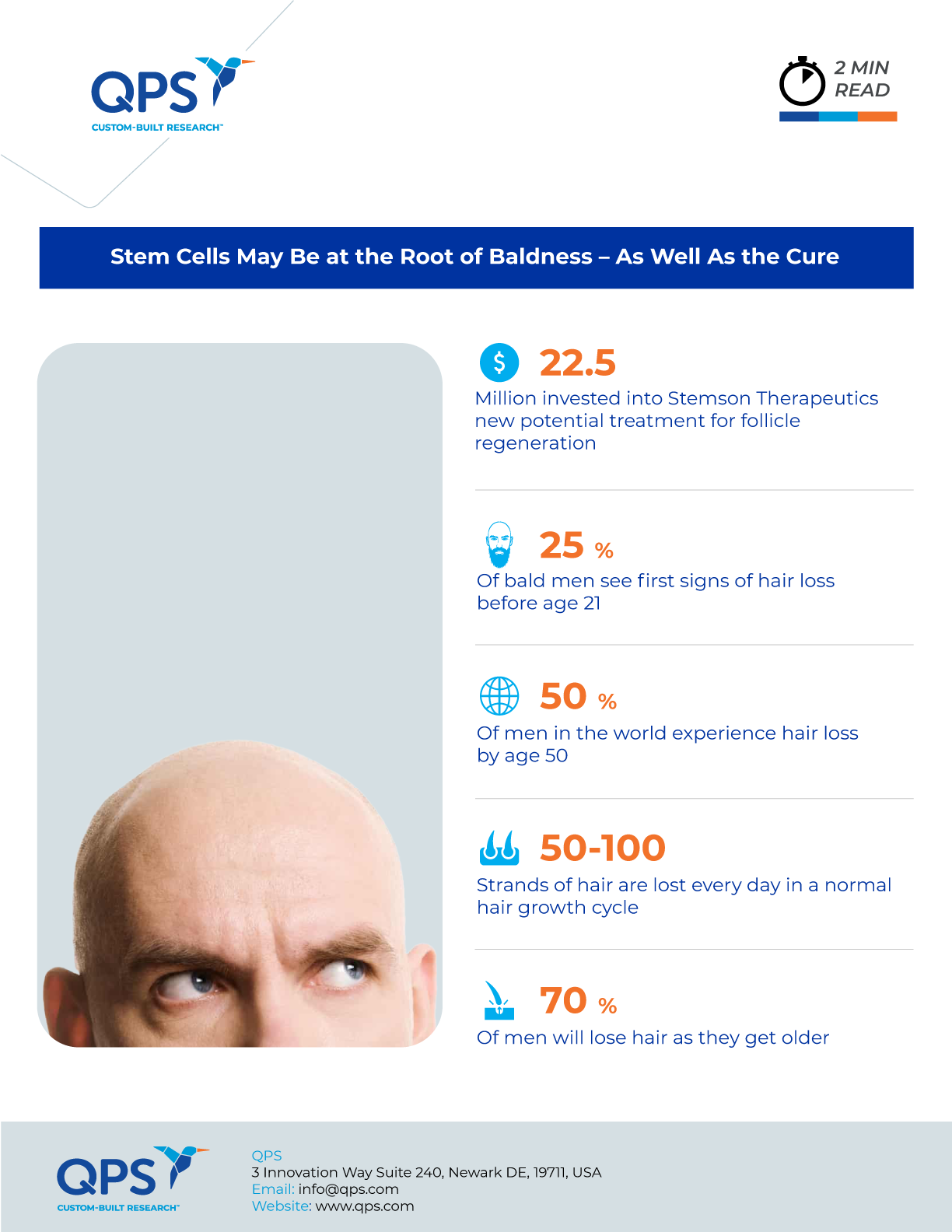
Aging, hormonal changes and disease can cause this cycle to stop, ending new hair growth. As with many other mysteries of aging, researchers wondered what exactly interrupts this cycle. A prevailing theory was that hair stem cells became “exhausted” and the cycle simply … stopped. Recent research, however, studied individual hair follicles in the ears of mice and demonstrated that, rather than dying in place, hair stem cells in aging mice simply … left. The cells transformed to squeeze through tiny holes in the follicle, then resumed their normal round shape and took off. This departure would explain the reduction in the size and functioning of the follicle, stopping new hair growth.
Developing stem cell therapy for hair loss is a robust area of research. Scientists are trying to harness the power of cell reprogramming — transforming any type of tissue into stem cells — to create, and then transplant, hair stem cells to restore hair growth. A few startups think they’ve achieved it.
Reprogramming and Reintroducing
Using the $22.5 million raised from investors including AbbVie, Stemson Therapeutics has developed a method for generating new follicles from patient cells using induced pluripotent stem (iPS) cells – tissue-specific cells, such as skin cells, that are lab-engineered to behave like embryonic stem cells with the ability to produce all types of cells in the body. The company reported that it hopes to “optimize our solution for human skin structure and environment so we can go into our first human clinical trial with high confidence for a positive outcome,” and that it is considering a robotic delivery system.
Meanwhile, the startup dNovo uses what it calls “direct reprogramming technology” to genetically engineer personalized hair stem cells from skin cells. In their research, scientists grafted the reprogrammed cells onto hair-deficient mice, which then sprouted a tuft of human hair at the grafting site. For some, the image of a patch of human hair growing on a bald mouse may be either amusing or disturbing – but for researchers it looked like proof of principle that induced hair stems cells can be used to grow hair.
dNovo founder and CEO Dr. Ernesto Lujan explained, “Direct reprogramming technology does not rely on generating pluripotent cells but instead directly converts one cell type into another (for example, in our case, skin cells to hair stem cells). This makes our system quick and scalable. We are further optimizing our technology platform and look forward to working with potential partners to bring our reprogramming system to the next stage.” The company reported in January that it had received $2.7 million in funding from multiple sources.
A Solution that Might Make the Cut
The last time a new hair loss drug gained regulatory approval was when Propecia® entered the market in 1992 – but with side effects, including decreased sex drive (among others), many don’t see the tradeoffs as worthwhile. As Geoff Hamilton, Stemson co-founder and CEO, noted in a letter to investors and patients, “Unfortunately, the biotech and pharmaceutical industry have not delivered new solutions nor is the R&D pipeline active in a meaningful way to give people suffering from hair loss the solutions they need. This needs to change.” Engineered stem cells may signal the beginning of this change.
Did you enjoy this blog post? Check out our other blog posts as well as related topics on our Webinar page.
QPS is a GLP- and GCP-compliant contract research organization (CRO) delivering the highest grade of discovery, preclinical and clinical drug research development services. Since 1995, it has grown from a tiny bioanalysis shop to a full-service CRO with 1,100+ employees in the U.S., Europe and Asia. Today, QPS offers expanded pharmaceutical contract R&D services with special expertise in neuropharmacology, DMPK, toxicology, bioanalysis, translational medicine and clinical development. An award-winning leader focused on bioanalytics and clinical trials, QPS is known for proven quality standards, technical expertise, a flexible approach to research, client satisfaction and turnkey laboratories and facilities. Through continual enhancements in capacities and resources, QPS stands tall in its commitment to delivering superior quality, skilled performance and trusted service to its valued customers. For more information, visit www.qps.com or email info@qps.com.