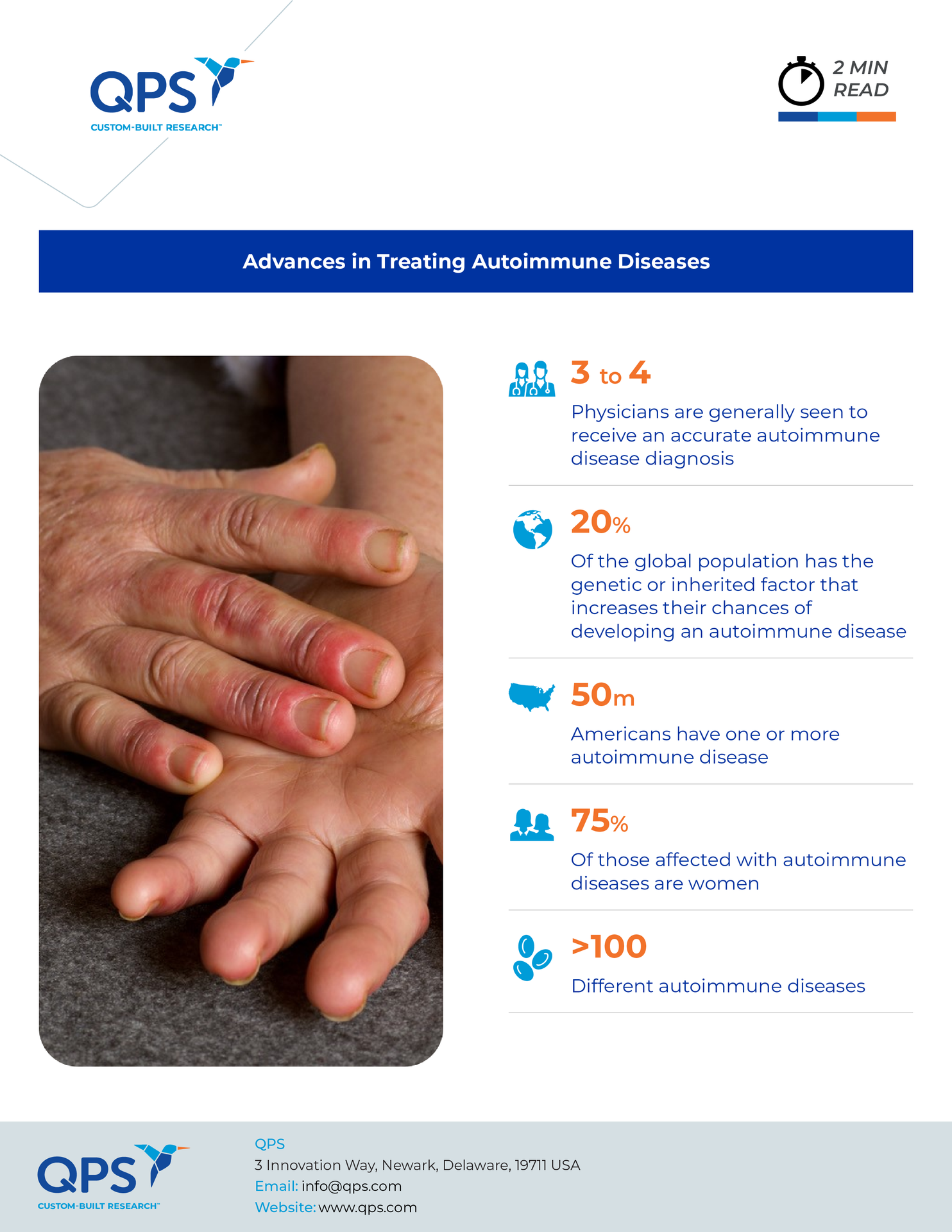
For decades, the battle against autoimmune diseases like type 1 diabetes, lupus, and multiple sclerosis has been an uphill struggle. Conventional treatments most often alleviate symptoms without addressing the underlying causes. However, recent advancements have sparked new hope for affected populations. Researchers are developing new therapies designed to restore what is known as immune tolerance, potentially revolutionizing autoimmune disease management.
The Quest for Immune Tolerance
The traditional approach to treating autoimmune diseases often involves suppressing the entire immune response, leaving individuals vulnerable to infections and cancers. However, the pursuit of immune tolerance offers a more promising path forward. By restoring the immune system’s ability to distinguish between self and non-self, researchers aim to quell autoimmune attacks without compromising overall immune function.
A successful therapy using this strategy could be life-changing. “The therapies that we have for these diseases are terrible,” said Maximilian Konig, a rheumatologist at Johns Hopkins University in Baltimore, Maryland. He noted that current therapies are not always effective, and even when they are, they often only offer modest improvement. Suggesting that new strategies could provide a cure is “almost blasphemy,” he said. However, research reports suggest that curative treatments could be on the horizon. “The only question is, what is the best approach?” he asks.
Nanoparticle Approach
Pere Santamaria, an immunologist at the University of Calgary in Canada, founded the biopharmaceutical company Parvus Therapeutics to develop nanomedicines to be used against autoimmune diseases. His strategy is founded on the idea that nanoparticles can be engineered to target and neutralize the rogue immune cells responsible for driving these diseases. The company hopes to conduct its first trial in humans within the year.
Leveraging the Liver
The liver plays a pivotal role in establishing immune tolerance by filtering blood carrying foreign antigens and cellular debris. Researchers led by Jeffrey Hubbell, a chemical engineer at the University of Chicago in Illinois, found that adding a special sugar tag to proteins can direct molecules to the liver, including antigens involved in multiple sclerosis. In published research, the team demonstrated that the antigen treatment reversed symptoms of a multiple sclerosis-like disease in mice, offering hope for effective treatments. Anokion, a company co-founded by Hubbell, has tested a similar approach in a Phase I trial and is moving ahead with a Phase II trial.
Going After B Cells
Meanwhile, other researchers are exploring diverse therapies directly targeting B cells, pivotal players in autoimmune diseases. Researchers led by Fabian Mueller at the University Hospital Erlangen in Germany have found success using chimeric antigen receptor (CAR) T-cell therapies, which equip T cells to target B cells. Based on early results from eight patients with lupus, four with systemic sclerosis, and three with idiopathic inflammatory myositis, the research team reported that all had improved symptoms and stayed in remission following treatment discontinuation.
B cells are also the focus for Aimee Payne, a dermatologist at Columbia University in New York City, who studies mucosal pemphigus vulgaris, a rare autoimmune skin disease. She is developing a chimeric autoantibody receptor (CAAR) T-cell therapy that targets specific disease-causing B cells but spares healthy B cells. Payne’s company, Cabaletta Bio, is currently testing CAAR T-cell therapies in patients.
The Road Ahead: Finding the Right Approach
With positive outcomes emerging from multiple immune tolerance strategies, researchers are cautiously optimistic about the potential for curative treatments for autoimmune diseases. As research and innovation in the field continue, Konig notes that “the beauty is that eventually, we’ll learn from the data what the right approach is.”
Did you enjoy this blog post? Check out our other blog posts as well as related topics on our Webinar page.
QPS is a GLP- and GCP-compliant contract research organization (CRO) delivering the highest grade of discovery, preclinical and clinical drug research development services. Since 1995, it has grown from a tiny bioanalysis shop to a full-service CRO with 1,100+ employees in the U.S., Europe and Asia. Today, QPS offers expanded pharmaceutical contract R&D services with special expertise in neuropharmacology, DMPK, toxicology, bioanalysis, translational medicine and clinical development. An award-winning leader focused on bioanalytics and clinical trials, QPS is known for proven quality standards, technical expertise, a flexible approach to research, client satisfaction and turnkey laboratories and facilities. Through continual enhancements in capacities and resources, QPS stands tall in its commitment to delivering superior quality, skilled performance and trusted service to its valued customers. For more information, visit www.qps.com or email info@qps.com.