QPS India
QPS Bioserve India Pvt. Ltd.
Registered Office:
Plot No. 47, IDA Balanagar
Hyderabad, TS 500037 India
CIN: U85110TG2004PTC044908
Clinical Studies Completed
0
Subjects Involved in Clinical Trials
0
Sq. ft. of Lab Space
0
Employees
0
QPS India Virtual Tour
Core Competencies
- North American standard Covid-19 safety measures in place in the lab and the clinic to protect our staff and all study subjects
- Global generic drug assay method development and validation team
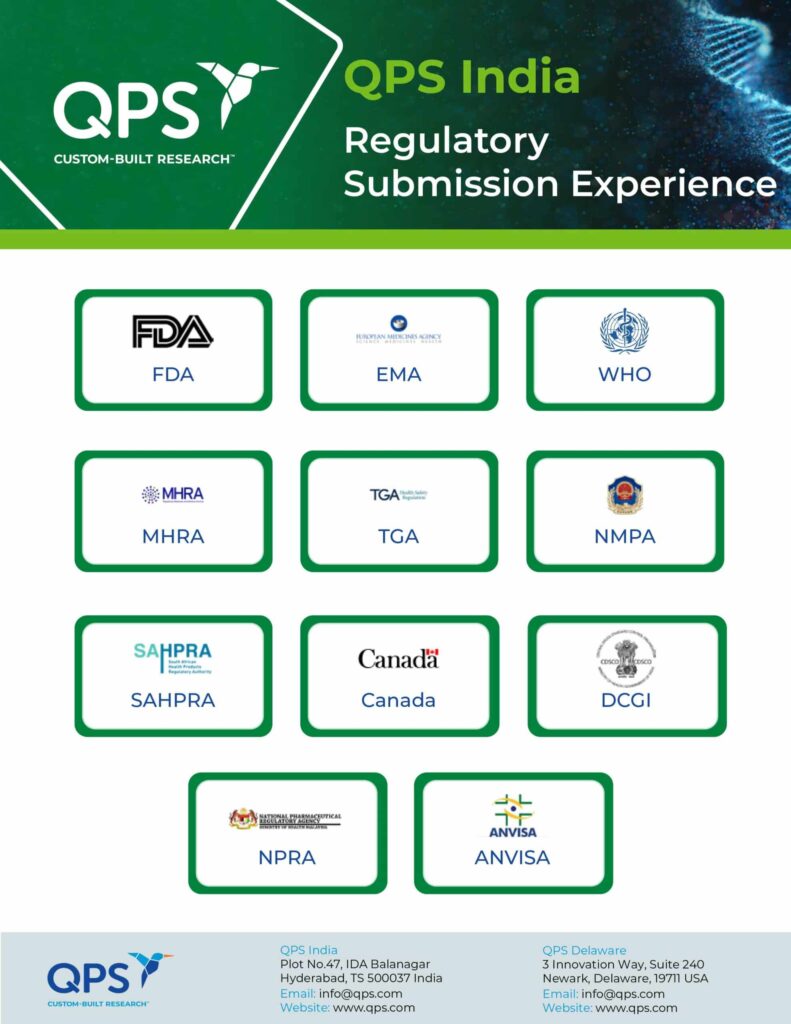
- Bioavailability / bioequivalence (BA/BE) studies to support new and generic drug registrations for Drug Controller General of India (DCGI) , US Food and Drug Administration (US FDA), UK Medicines and Healthcare products Regulatory Agency (MHRA), European Medicines Association (EMA) , Therapeutic Products Directorate (TPD), World Health Organization (WHO) , and Medicines Control Council (MCC)
- Early Phase clinical development (Phase I → Phase IIa), including PK/PD
- Late Phase clinical development (Phase III → Phase IV) studies to support new and generic drugs
- Bioanalysis for small molecules using LC-MS/MS
- Sample receipt from any global location
- Project Management
- Dedicated, full-time Clinical Research Associates (CRA) for clinical monitoring.
- Clinical Data Management; including eCRF design, data entry, query management, data cleaning, SAS programming, SDTM and ADaM datasets to meet current CDISC standards.
- Medical writing; including study synopsis, protocol, investigator’s brochure, informed consent forms, case report forms, ICH E3 integrated clinical reports, statistics, and eCTD publication.
- Pharmacokinetics and biostatistics using WinNonlin and SAS.
- Quick turnover-time to support first-to-file studies.
- Successful recruitment of healthy volunteers and patient study subjects across numerous GCP sites.
- Ability to successfully recruit study subjects for partial-replicate and replicate designs with long washouts and ensure subject panel completion, even with stringent Covid-19 safety procedures in place.
Facility
- 138 clinic beds
- Designated ICUs (with fully inventoried crash carts) in every clinical dosing area to protect study subjects against unforeseen AEs and SAEs.
- 4 bed emergency unit to handle adverse events (AEs)
- 3 wet laboratories
- 10 LC MS/MS systems (triple quadrupole mass spectrometers – TQMS)
- Over 1,000 bioanalytical, validated assays
- Capacity to analyze over 250,000 samples per year
- On-site, access controlled pharmacy
- ISO 15189-accredited clinical laboratory
- On-site canteen for study participants’ meal preparation
- Automated temperature monitoring for freezers and refrigerators
- Dual-level power backup system for the facility
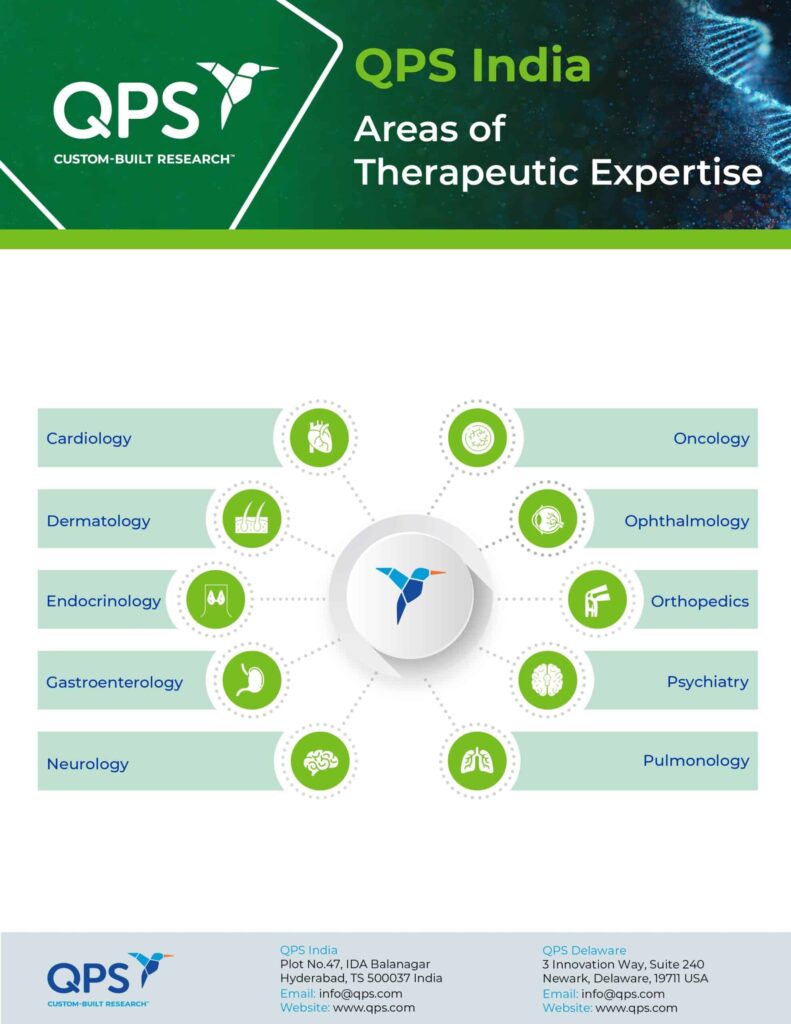
- 500 hospital network locations for patient studies in oncology, dermatology, ophthalmology, metabolism, gastroenterology, neurology, and respiratory diseases
- Study subject tracking system
- A database including over 12,000 healthy potential study subjects
Achievements
- 40 regulatory inspections in 18 years
- 15 successful US FDA inspections (latest in August 2023)
- 12 US FDA inspections with no 483’s
- 3 consecutive 483-free inspections since 2021
- 9 successful DCGI inspections (latest in April 2018)
- 2 successful WHO inspections (latest in October 2017)
- 1 successful EMA inspection (latest in April 2015)
- Additional inspections by UK MHRA and NPRA
- Approved by ANVISA to conduct studies for submission in Brazil
People
- Highly experienced management staff with over 200 combined years of clinical BA/BE experience.
- Highly experienced team, including GMP, GCP, GLP, and GEP areas.
Experience
- Created and executed over 1,500 generic drug study designs.
- Successfully completed over 1,600 clinical PK studies (80% of which advanced to regulatory approval).
- Submitted 800+ studies to various regulatory agencies.
- Over 200 first-to-file studies.
- Expertise in multi-drug administration routes; including oral, intravenous (IV), intramuscular (IM), subcutaneous (SC), and topical.
- Experience across a broad range of patient populations; including, CNS (Neuropsychiatry), Cardiology, Endocrinology, Gastroenterology, HIV/Infections Diseases, Nephrology, Oncology, Opthalmology, Pulmonology, etc.
- Ability to dose controlled substances at the QPS Missouri USA site, while catering to all the back end services out of QPS India.
QPS India
- QPS India has achieved another significant milestone by successfully completing a scheduled inspection by the UK Medicines and Healthcare products Regulatory Agency (MHRA) in November 2024. The inspection was a systems & processes audit resulting in the site approval (valid for at least next 2 years) for all studies conducted at QPS India and submitted by sponsors for UK regulatory agency approval.
- QPS India, an American GLP/GCP-compliant Contract Research Organization (CRO) in India, providing first class full service CRO offerings, catering to all phases of drug development and recruiting healthy subjects as well as patient populations.
- QPS India was established in 2004 and is located in Hyderabad, India, with 36,000 square feet of facility space and 175+ employees.