
Moo vs. Flu: Seasonal Flu Antibody Therapy Produced in Cows
Humans have used cows in vaccine development dating back to 1770 and the origins of the smallpox vaccine. In more modern medicine, transgenic cows have

Humans have used cows in vaccine development dating back to 1770 and the origins of the smallpox vaccine. In more modern medicine, transgenic cows have
The World Health Organization reports that around 13 percent of the world’s population is classified as “obese.” Today, medical experts know that obesity is much more than

In evolutionary terms, chimpanzees are humans’ closest living relatives. But how close are we, really? Science Daily reports that researchers at Lund University in Sweden
Despite being one of the world’s most common inherited blood disorders, sickle cell disease has few treatment options, even with the approval of three new
The recent release of findings from a small Phase 1 clinical trial not only gives hope to families affected by an ultra-rare childhood disease, but

CAR-T is an immunotherapy that is currently used to treat blood cancers and is in clinical trials to treat other cancers as well. This treatment

While research on potential therapeutic applications of mRNA has been underway for more than a decade, the success of the Pfizer and Moderna COVID-19 vaccines
Biotech start-up Ring Therapeutics, founded by Flagship Pioneering in 2017, hopes to transform gene therapy by using a new class of vector — anelloviruses –

An estimated 650 million adults live with obesity today, which puts those individuals at increased risk for some of the leading causes of death worldwide,
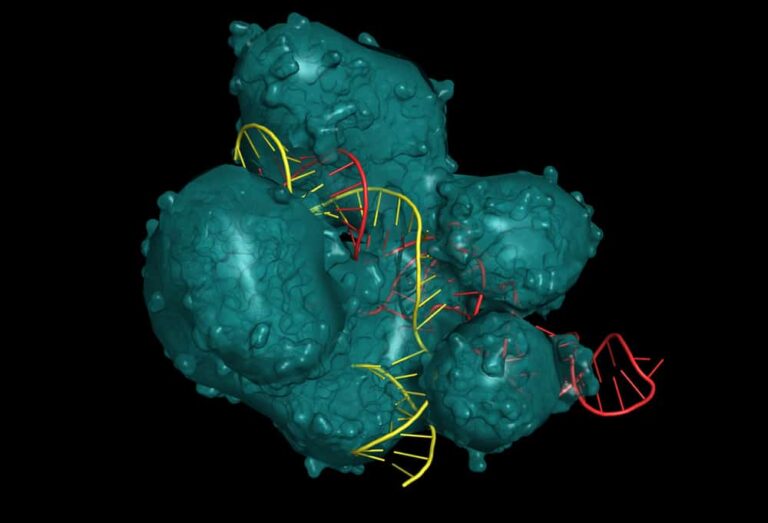
Transthyretin amyloidosis (ATTR amyloidosis) is a rare but fatal disease marked by clumping and accumulation of mutated, misfolded transthyretin proteins in peripheral nerves and cardiac

Depending on your perspective, giraffes are either the world’s most graceful animal or a baffling, doe-eyed sloppy eater. (This could depend largely on whether you’re
As a hereditary trait, eye color tends to be an introductory lesson to human genetics. Think back to high school biology class, which often covers

Humans have used cows in vaccine development dating back to 1770 and the origins of the smallpox vaccine. In more modern medicine, transgenic cows have

The World Health Organization reports that around 13 percent of the world’s population is classified as “obese.” Today, medical experts know that obesity is much more than

In evolutionary terms, chimpanzees are humans’ closest living relatives. But how close are we, really? Science Daily reports that researchers at Lund University in Sweden

Despite being one of the world’s most common inherited blood disorders, sickle cell disease has few treatment options, even with the approval of three new

The recent release of findings from a small Phase 1 clinical trial not only gives hope to families affected by an ultra-rare childhood disease, but

CAR-T is an immunotherapy that is currently used to treat blood cancers and is in clinical trials to treat other cancers as well. This treatment

While research on potential therapeutic applications of mRNA has been underway for more than a decade, the success of the Pfizer and Moderna COVID-19 vaccines

Biotech start-up Ring Therapeutics, founded by Flagship Pioneering in 2017, hopes to transform gene therapy by using a new class of vector — anelloviruses –

An estimated 650 million adults live with obesity today, which puts those individuals at increased risk for some of the leading causes of death worldwide,

Transthyretin amyloidosis (ATTR amyloidosis) is a rare but fatal disease marked by clumping and accumulation of mutated, misfolded transthyretin proteins in peripheral nerves and cardiac

Depending on your perspective, giraffes are either the world’s most graceful animal or a baffling, doe-eyed sloppy eater. (This could depend largely on whether you’re

As a hereditary trait, eye color tends to be an introductory lesson to human genetics. Think back to high school biology class, which often covers
Delaware Technology Park,
3 Innovation Way,
Newark, DE 19711
"*" indicates required fields